Abstract
Background
Up to 50% of the patients in the intensive care unit (ICU) require mechanical ventilation, with 20% requiring the use of a ventilator for more than 7 days. More than 40% of this time is spent weaning the patient from mechanical ventilation. Failure to wean from mechanical ventilation can in part be attributable to rapid onset of diaphragm atrophy, barotrauma, posterior lobe atelectasis, and impaired hemodynamics, which are normally improved by maintaining a more natural negative chest pressure. The authors have previously shown that laparoscopic implantation of a diaphragm pacing system benefits selected patients. They now propose that an acute ventilator assist with interventional neurostimulation of the diaphragm in the ICU is feasible and could facilitate the weaning of ICU patients from mechanical ventilation. Natural orifice transluminal endoscopic surgery (NOTES) has the potential to expand the benefits of the diaphragm pacing system to this acute patient population by allowing it to be performed at the bedside similarly to insertion of the common gastrostomy tube. This study evaluates the feasibility of this approach in a porcine model.
Methods
Pigs were anesthetized, and peritoneal access with the flexible endoscope was obtained using a guidewire, needle knife cautery, and balloon dilation. The diaphragm was mapped using a novel endoscopic electrostimulation catheter to locate the motor point (where stimulation provides complete contraction of the diaphragm). An intramuscular electrode then was placed at the motor point with a percutaneous needle. The gastrotomy was managed with a gastrostomy tube.
Results
Four pigs were studied, and the endoscopic mapping instrument was able to map the diaphragm to identify the motor point. In one animal, a percutaneous electrode was placed into the motor point under transgastric endoscopic visualization, and the diaphragm could be paced in conjunction with mechanical ventilation.
Conclusions
These animal studies demonstrate the feasibility of transgastric mapping of the diaphragm and implantation of a percutaneous electrode for therapeutic diaphragmatic stimulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Natural orifice transluminal endoscopic surgery (NOTES) may emerge as an alternative to standard laparoscopic or open abdominal procedures. However, the infancy of the emerging field translates to a relative paucity of procedures, which may limit the incorporation of NOTES into routine clinical practice. Unlike laparoscopic cholecystectomy, which capitalizes on its high volume to propel the emerging field of laparoscopy, no high-volume NOTES procedure exits. Our research team has shown that laparoscopic implantation of a diaphragm pacing stimulation (DPS) system can strengthen and condition the diaphragm to facilitate weaning of patients with high spinal cord injury off mechanical ventilators to breathe using their own muscles with the DPS [12].
Our goal was to design a new operation and a new paradigm for treating patients using these neurostimulation and NOTES technologies from the ground floor up. Instead of trying to duplicate a laparoscopic operation, a unique application for NOTES was developed. Secondary benefits of this project may translate to a high-volume use of NOTES that will stimulate academic and industrial interest in NOTES and accelerate acceptance of endoscopic surgery, resembling the effects that laparoscopic cholecystectomy had on the overall field of laparoscopy.
The large group of patients that may benefit from this proposed initial application of NOTES is intensive care unit (ICU) patients using ventilators. Almost half of critically ill ICU patients require mechanical ventilation, with 20% requiring the use of a ventilator for more than 7 days. More than 40% of a patient’s time in the ICU is spent weaning from mechanical ventilation. Failure to wean from mechanical ventilation can in part be attributable to rapid onset of diaphragm atrophy, barotrauma, posterior lobe atelectasis, and impaired hemodynamics. We believe these negative aspects of mechanical ventilation may be improved with the use of temporary electrodes on the diaphragm.
Because many ICU patients already have bedside endoscopy performed for percutaneous endoscopic gastrostomies (PEG), NOTES may be a logical segue to facilitate DPS implantation. Current DPS implantation is performed laparoscopically in operating rooms, and although bedside laparoscopy is performed in ICUs, it still is not as accepted or as easy as bedside endoscopy.
The objectives of this study were to design the necessary tools to enable NOTES mapping of the diaphragmatic motor point (to locate where stimulation provides maximal diaphragm contraction), to develop temporary percutaneous electrodes and an implant method, and to evaluate the operative feasibility of placing temporary pacing wires in the diaphragm transgastrically during a PEG in a porcine model. The discussion also provides the background on the use of temporary diaphragm pacing, which may offer a paradigm shift in the management of ventilator patients.
Methods
All animal experiments were completed in accordance with preauthorized consent obtained from the Case Western Reserve University Institutional Animal Care and Use Committee. Four healthy female domestic farm pigs weighing 25 ± 5 kg were obtained from a local vendor (Pineview Farms, Valley City, OH, USA). The animals were removed from bedding 72 h before the procedure. They were allowed water but no food for 24 h before the procedure. All the animals were sedated with 8 mg/kg of tiletamine HCl and zolazepam (Telazol; Fort Dodge Animal Health, Fort Dodge, Iowa) injected intramuscularly and intubated with a 6-Fr endotracheal tube. Peripheral intravenous access was obtained. Supplemental anesthesia, if required, was induced with 25 mg/kg of thiopental sodium (Pentothal; Abbot Laboratories, North Chicago, Illinois). Isoflurane 0.5% to 2% (AErrane; Baxter, Deerfield, IL, USA) was administered by inhalation via the endotracheal tube. Cardiac and pulse oximetry ensured normal physiologic response to anesthetic agents. The animals were mechanically ventilated at a tidal volume of 15 to 20 ml/kg with 100% oxygen at 12 respirations/min.
Transgastric access was achieved using our described technique with a standard single-channel video endoscope (Olympus EVIS Type 100 Q140, Olympus, Tokyo, Japan) [9, 17]. Using a Seldinger technique, a guidewire was placed in the gastric lumen at a standard anterior site on the abdominal wall for a PEG. The endoscope and guidewire then were brought out through the mouth, and the endoscope was reinserted alongside the guidewire. A gastrotomy was performed with a combination of needle knife cautery used to make the initial incision at the site of the guidewire, followed by endoscopic balloon dilation to enlarge the gastrotomy. The endoscope was advanced into the peritoneal cavity for visualization of the porcine hemidiaphragms.
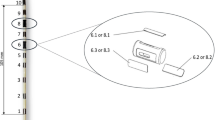
To achieve diaphragm activation, the intramuscular electrodes require proper placement within the diaphragm muscle. For optimal activation of the muscle, the electrodes must be placed in close proximity to the phrenic nerve motor point (the point at which the phrenic nerve inserts into diaphragm). We previously described this point in a cadaver study and determined that there is no one anatomic landmark for identifying it [10]. Therefore, the location for implantation of the electrode needs to be identified by stimulating the diaphragm with a probe electrode (Synapse Biomedical, Oberlin, OH, USA) and qualitatively assessing the strength of the diaphragm contraction (Fig. 1). In our spinal cord and amyotrophic lateral sclerosis (ALS) clinical studies with atrophied or partially denervated diaphragms, we quantitatively assess the strength of the contraction by measuring changes in abdominal pressure [11]. Proximity to the motor point is identified by increasing abdominal pressure. With relatively normal diaphragms, qualitative assessment alone through visual observation of the diaphragm contraction may be adequate for the placement of temporary electrodes.
Currently used laparoscopic mapping probe being used to identify the motor point on the right diaphragm. The probe has a suction port attached by vacuum so that the contact electrode at its tip can be attached noninvasively to the surface of the muscle receiving a stimulus from an external computer-controlled stimulator.
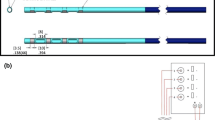
To facilitate conversion of the laparoscopic motor point mapping process to a NOTES procedure, a prototype endoscopic mapping instrument was developed to provide a mechanism for introducing a temporary stimulating electrode through a standard 2.8-mm endoscope lumen. The stimulating tip consists of a 9-mm length of 304 stainless steel tubing, which is plasma welded on one end to form a bullet tip. A length of 14-strand 316 stainless steel wire is crimped into the other end of the stimulating tip, then threaded through a short 15-mm length of thin wall silicone tubing and through the 170-mm length of polyetheretherketone polymer (PEEK) tubing used as a body of the instrument. The silicon tubing is expanded and installed between the stimulating tip and the PEEK tubing to provide a flexible interface that will not interfere with the stimulated diaphragm contraction. A stainless steel ferrule crimps the wire to the other end of the PEEK tubing to provide a convenient location for connecting a clip lead attached to a standard NeuRx RA4 Percutaneous Neuromuscular Stimulator (Synapse Biomedical, Oberlin, OH, USA).
With a completed prototypical NOTES mapping system designed, attention was turned to modifying the chronic human electrode to provide temporary diaphragmatic pacing. Findings have shown the electrode used in our chronic human studies to be very dependable, with more than 35 years of cumulative use in 32 patients. A total of 130 electrodes have been implanted in human diaphragms with no mechanical failures or inadvertent migration removal from their implanted sites in the diaphragm.
The modified temporary stimulating lead consists of a helically wound length of 7-strand 316 stainless steel wire insulated with a perfluoralkoxy coating. The stimulating tip is made by de-insulating the last 9 mm of the wound lead. A barb is formed by bending the de-insulated portion so that when implanted in the muscle tissue, the barb will “catch” on the fibers as the introducing needle is withdrawn. Because this lead is intended for short-term use (several days to several weeks) and withdrawn without further surgical intervention, no additional polypropylene barbs are added, as with the use of the chronic diaphragm pacing electrode. This version of the lead also does not have the polypropylene core along its full length or a redundant wind of the 7-strand wire that the chronic version of the lead has.
With the design of the components complete, the equipment and technique were evaluated in a series of experimental animals. After diaphragm assessment, the gastrotomy was managed by attaching a standard pull PEG tube to the guidewire left in place during the NOTES procedure. The PEG was withdrawn back through the gastrotomy, leaving the internal mushroom bumper in the gastric lumen. The lumen of the stomach was distended with 500 ml of diluted india ink solution, evaluated for leakage, and subsequently sacrificed and analyzed.
Results
Four pigs were studied successfully with NOTES. The left and right diaphragm could be adequately visualized in all the pigs with endoscopic retroflexion. The gastric exit site chosen for the future PEG allowed easy visualization of both the right and left diaphragm. The prototype endoscopic mapping device allowed stimulation of the diaphragm with qualitative assessment of diaphragm contraction in all four pigs (Fig. 2). The visualization and stimulation were comparable with the laparoscopic visualization of the spinal cord and ALS trials, although the view was upside down. In one pig, the prototype electrode was placed under transgastric endoscopic visualization into one hemidiaphragm at the mapped motor point. The electrode was in a standard spinal needle introduced percutaneously through the lateral abdominal wall.
Once orientation was obtained with the inverted and transposed visualization, the needle was introduced in a parallel fashion into the diaphragm muscle, then withdrawn, allowing the electrode, because of the barb, to stay in the diaphragm (Fig. 3). This electrode then was attached to the DPS stimulator, and the diaphragm was paced synchronously with the ventilator. Endoscopic quantitative assessment of the strength of diaphragmatic contraction was completed.
After completion of the case and closure of the gastrotomy with a PEG, the diaphragm contraction with stimulation by the DPS system could be felt. After the animals had been killed, no extravasation of india ink at the site of the gastrotomy managed by the PEG was observed.
Discussion
The findings of this study show that the use of NOTES is feasible in a porcine model using a novel application of diaphragm pacing with patients on mechanical ventilation in an ICU. Assessing whether this concept deserves further study involves three separate issues: NOTES, diaphragm pacing, and acute diaphragm pacing in ICU patients. The NOTES approach is an extension of the benefits seen with the laparoscopic revolution of the 1990s. The NOTES procedure will have a role in patient care in the future and is not an issue in the concept of transgastric diaphragm pacing [7, 15, 16]. The development of a large-volume NOTES procedure will drive industry to develop the technology that will make NOTES a standard option in our management of patients.
Diaphragm pacing stimulation knowledge has reached a critical mass of knowledge in the small orphan disease populations for which it currently is being used. Of the 11,000 new patients with spinal cord injuries in the Unites States, only approximately 300 have intact phrenic nerves and require long-term ventilation. Currently, the DPS system has a 96% success rate in providing patients with an adequate tidal volume using their own stimulated diaphragms for breathing without the use of a ventilator [3, 4]. This same technology also is showing promising results for the 6,000 newly diagnosed amyotrophic lateral sclerosis (ALS or Lou Gehrig’s disease) cases in the United States, allowing the patients to maintain diaphragmatic strength and decreasing the rate of decline in their forced vital capacity, which should lengthen their time until mechanical ventilation [14]. The ability to identify the motor point of the diaphragm and implant electrodes laparoscopically is past the learning curve. It is a proven viable option that is not an issue in this concept [13].
Therefore, two of the three aspects (NOTES and diaphragm pacing) appear to have merit in the assessment of this study. The last assumption, that diaphragm pacing of patients using ventilators in the ICU will have a role in the future, is the one that requires a paradigm shift in the way we manage patients. At any given time, approximately 1.6 million patients in the United States are receiving mechanical ventilation. More than 100,000 tracheostomies are performed annually for prolonged weaning of patients on mechanical ventilators [6]. With the average cost of an ICU day at $4,000, it has been estimated that prolonged mechanical ventilation accounts for 37% of all ICU costs ($25 billion).
Cost is not the only untoward factor in prolonged mechanical ventilation. Ventilator-associated pneumonia has a direct relationship to the time a patient spends on mechanical ventilation, and to the morbidity and mortality of patients in ICUs. We believe decreasing the length of time a patient spends on a ventilator will decrease the incidence of ventilator-associated pneumonia. But is there evidence that diaphragm pacing could help patients in the ICU using mechanical ventilation?
Animal studies have shown that within 12 h of mechanical ventilation, diaphragm atrophy can be identified with reduced diaphragm mass by 48 h [5]. In baboon studies, short durations of mechanical ventilation led to a significant impairment of diaphragmatic strength and endurance [1]. Ventilator-induced diaphragmatic dysfunction also has been described in humans [19]. Functional electrical stimulation (FES) to extremity muscles in patients on mechanical ventilation in ICUs has been shown to improve muscle strength and the ability to transfer out of bed [20]. In a case report of a spinal cord patient who had a phrenic nerve cuff electrode system for ventilation, with only one side functional, the authors concluded that FES of only 30 min a day could suppress pathologic diaphragmatic attenuation and preserve diaphragm thickness and function [2]. Until development of the DPS system, there has not been a way to provide temporary electrical stimulation to the diaphragm.
Mechanical ventilation preferentially ventilates the anterior lobes of the lung, which predisposes the patient to atelectasis and subsequent pneumonia in the posterior lobes [18]. Diaphragm pacing in synchronization with a ventilator would improve posterior lobe ventilation and subsequently decrease atelectasis and ventilator-associated pneumonia. Researchers have already shown that respiratory control by diaphragm pacing is hemodynamically superior to mechanical ventilation because it decreases the intrathoracic pressure, allowing increased central venous return [8].
In conclusion, the reported animal studies support the concept that incisionless bedside implantation of a DPS system is feasible. We have identified a series of steps that now need to be studied before human trials. The electrode implant needle will be further refined for human use so that it is longer and has the ability to angulate to more closely match our current laparoscopic implant instrument. Further animal studies are under way to assess the placement of bilateral electrodes. Chronic animal studies aim to identify the possibility of the electrodes becoming infected, and to assess the ability to remove these percutaneous wires. In the chronic survival animal model, during necropsy, leaks will be assessed by looking for any evidence of contamination such as abscesses, and by culturing the pacing wires to assess for clinically relevant contamination. We are already studying the use of diaphragm pacing in synchronization with the ventilator in our spinal cord protocols. This will allow us to gain the experience to begin a feasibility study to assess the safety of the DPS system in humans.
The initial studies of the DPS system for acute pacing in the ICU will be conducted laparoscopically at the time of a tracheostomy and PEG. We then will easily transition it to be performed with NOTES. In the future, if the paradigm of maintaining diaphragm strength with pacing prevents the need for a tracheostomy, the classification of a patient in an ICU will change from the need for a “trach” and a PEG to the need for a PEG and DPS wires.
References
Anzueto A, Peters JI, Tobin MJ, de los Santos M, Seidenfield J, Moore G, Cox WJ, Coalsons J (1997) Effects of prolonged controlled mechanical ventilation on diaphragmatic function in healthy adult baboons. Crit Care Med 25: 1187–1190
Ayas NT, McCool FD, Gore R, Lieberman SL, Brown R (1999) Prevention of human diaphragm atrophy with short periods of electrical stimulation. Am J Respir Crit Care Med 159: 2018–2020
DiMarco AF, Onders RP, Ignangi AI, Kowalski KE,Stephan S, Mortimer JT (2005) Phrenic nerve pacing via intramuscular diaphragm electrodes in tetraplegic subjects. Chest 127: 671–677
Dimarco AF, Onders RP, Kowalski KE, Miller ME, Ferek S, Mortimer JT (2002) Phrenic nerve pacing in a tetraplegic patient via intramuscular diaphragm electrodes. Am J Respir Crit Care Med 166: 1604–1606
Gayan-Ramirez G, Decramer M (2002) Effects of mechanical ventilation on diaphragm function and biology. Eur Respir J 20: 1579–1586
HCUPnet, Healthcare Cost and Utilization Project (2006) Agency for Healthcare Research and Quality, Rockville, MD. Retrieved January 24, 2006 at http://www.ahrq.gov/HCUPnet
Hochberger J, Lawade W (2005) Transgastric surgery in the abdomen: the dawn of a new era? Gastrointest Endosc 62: 293–296
Ishii K, Kurosawa K, Koyanagi H, Nakano K, Sakakibara N, Sato I, Noshior M, Ohsawa M (1990) Effects of bilateral transvenous diaphragm pacing on hemodynamic function in patients after cardiac operations: experimental and clinical study. J Thorac Cardiovasc Surg 100: 108–114
Marks J, Rosen M, McGee M, Chak A, Onders R, Faulx A, Ignagni A, Schmoisch S, Ponsky J (2006) A novel technique for management of endoscopic gastrotomy following natural orifice transvisceral endoscopic surgery. Surg Endosc 20: s287
Onders RP, Aiyar H, Mortimer JT(2004) Characterization of the human diaphragm muscle with respect to the phrenic nerve motor points for diaphragmatic pacing. Am Surg 70: 241–247
Onders RP, DiMarco AF, Ignagni AI, Aiyer H, Mortimer JT (2004) Mapping the phrenic nerve motor point: the key to a successful laparoscopic diaphragm pacing system in the first human series. Surgery 136: 819–826
Onders RP, DiMarco AF, Ignagni AR, Mortimer JT (2003) Laparoscopic placement of diaphragm pacing systems in human subjects. Laparosc SLS Rep 2: 22–23
Onders RP, Ignagni AI, DeMarco AF, Mortimer JT (2005) The learning curve of investigational surgery: lessons learned from the first series of laparoscopic diaphragm pacing for chronic ventilator dependence. Surg Endosc 19: 633–637
Onders RP, Ignagni AR, Katirji B, Schilz R, Elmo MJ (2006) Early results of laparoscopic motor point diaphragm pacing in amyotrophic lateral sclerosis: can exogenous electrical stimulation impact respiratory failure? Amyotrophic Lateral Sclerosis Other Motor Neuron Dis 6: 142–143
Ponsky JL (2005) Gastroenterologists as surgeons: what they need to know. Gastrointest Endosc 61: 454
Rattner D, Kalloo A (2006) ASGE/SAGES Working Group on Natural Orifice Translumenal Endoscopic Surgery. Surg Endosc 20: 329–333
Rosen M, McGee M, Marks J, Chak A, Onders R, Faulx A, Ignagni A, Schomisch S, Ponsky J (2006) Optimizing peritoneal access for natural orifice transvisceral endoscopic surgery (NOTES). Surg Endosc 20: s364
Rouby J (2004) Optimizing lung aeration in positive end-expiratory pressure. Am J Respir Crit Care Med 170: 1039–1040
Vassilakopoulos T, Petrof BJ (2004) Ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med 169: 336–341
Zanotti E, Felicetti G, Maini M, Fracchia C (2003) Peripheral muscle strength training in bed-bound patients with COPD receiving mechanical ventilation: effect of electrical stimulation. Chest 124: 292–296
Acknowledgment
Case Western Reserve University, University Hospitals of Cleveland, Raymond P. Onders M.D., and Anthony Ignagni have the intellectual property of the devices used or equity in Synapse Biomedical, which manufactured the diaphragm pacing technology used in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented at the 2006 Scientific Session of the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) April 2006, Dallas, TX, USA
Rights and permissions
About this article
Cite this article
Onders, R., McGee, M.F., Marks, J. et al. Diaphragm pacing with natural orifice transluminal endoscopic surgery: potential for difficult-to-wean intensive care unit patients. Surg Endosc 21, 475–479 (2007). https://doi.org/10.1007/s00464-006-9125-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-006-9125-4