Abstract
Background
Although pediatric flexible-endoscopic evaluation of swallowing (FEES) has developed into a standard in dysphagia diagnostics, there are no valid protocols and procedures for children available to date.
Objective
This systematic PROSPERO-registered review aimed to identify implementation protocols for pediatric FEES described in research studies, and to analyze them in detail concerning procedural steps, equipment, and reported outcome.
Methods
Included were all studies reporting a pediatric FEES protocol for children aged 0–18 years, if they described at least two criteria defined in advance. The databases MEDLINE and CINHAL were searched systematically from January 2000 to February 2021. Risk of bias for included studies was assessed using the National Institutes of Health (NIH) quality assessment tool for observational cohort and cross-sectional studies. A narrative synthesis of the FEES protocols was conducted and the results compared in tabular form.
Results
In total 22 studies were included, reporting on FEES in 1547 infants, children, and adolescents with a wide range of diagnoses. It was possible to identify protocols related to all age groups in general as well as to particular groups such as breastfed or bottle-fed infants. None of the included studies demonstrated a good methodological quality; all studies had missing data. Uniform implementation for sub-groups could not be determined. The reported outcome of FEES examinations could not be compared.
Discussion
None of the included studies showed good methodological quality and a significant amount of data were missing; the review still offers a systematic basis for future research to close the serious gap in the area of pediatric FEES. A proposal is made for a minimum requirement for pediatric FEES protocols in scientific studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Flexible-endoscopic evaluation of swallowing (FEES) is a feasible and safe instrumental swallowing assessment procedure in children of all ages [1]. Langmore [2] recently published a historical review of FEES, highlighting the increasing use in children. The benefits are: the identification of anatomical abnormalities, the ability to assess the exact diet with food and liquids rather than barium in the child's preferred position, and the opportunity to examine while breastfeeding [2, 3]. Miller et al. [3] and Miller and Willging [1] recently published detailed protocols for carrying out pediatric FEES. These contain the classic FEES procedure according to Langmore [4] and a broad description of the types of swallowing modifications including compensatory strategies that can be utilized during FEES. Recommendations for the procedures in specific populations are given, however, valid scales for uniform evaluation are missing.
A recent systematic review [5] stated that FEES protocols for the adult population, especially for patients with a neurogenic main emphasis, are very well developed and well researched. Yet, even those protocols contain disagreements and inaccuracies. A systematic review of quantitative instrumental swallowing assessment in children [6] was unable to include a single study of pediatric FEES from the past 20 years due to methodological weaknesses in the available studies.
Based on these shortcomings, the aim of this review was to (i) summarize the implementation protocols for pediatric FEES described in research studies and (ii) analyze the protocols in detail with regard to procedural steps, equipment, and reported outcomes.
The primary research questions are “What implementation protocols for pediatric FEES are described in scientific studies, including technical and other equipment, and bolus texture and coloring?” and “What FEES-based outcomes concerning swallowing pathologies are reported and which scales are used to ensure objective classification? “ The secondary research question is “Are implementation protocols able to be identified for certain sub-groups and what factors are detectable that make up these subgroup protocols?”.
Methods
Search Strategy and Quality Assessment
This systematic review was registered on PROSPERO (CRD42021247396) and carried out roughly based on the “Preferred Reporting Items for Systematic Reviews and Meta-analyses Protocol” (PRISMA-P [7]). The MEDLINE and CINHAL databases were searched systematically from January 2000 to February 2021 using medical subject headings (MeSH) and keywords (Table 1). All eligible abstracts were screened for inclusion and exclusion criteria (Table 2). A manual search in the reference lists of the included articles was carried out to identify additional studies. Two reviewers (JZ and SK) independently evaluated the full texts for eligibility. An agreement was reached through discussion.
Inclusion and Exclusion Criteria
All original scientific journal articles published in English that reported on a FEES protocol for the detection of dysphagia in children and described at least two of the predefined criteria for accurate performance were included (Table 2). There were no restrictions on study design.
Risk of Bias and Quality Assessment
The risk of bias for each study was assessed with the National Institutes of Health (NIH) quality assessment tool for observational cohort and cross-sectional studies [8] (Table 3). One reviewer (JZ) carried out the assessment and one checked the results (SK). Disagreement was solved by discussion.
Data Synthesis
A narrative synthesis of the FEES protocols from the included studies was prepared roughly based on the SWiM (synthesis without meta-analysis) reporting guideline [9]. One reviewer extracted data from the studies (JZ) and three reviewers (TF, J-CK, SHK) checked the extracted data.
The modifiers (i) sample, (ii) FEES implementation, (iii) FEES equipment, and (iv) FEES outcome were transferred into tables. To ensure comparability of the data, they were standardized as much as possible. Children's ages were converted to months, and missing means and standard deviations were calculated where data from the studies allowed. The FEES procedural steps reported in the study protocols were summarized in standard terms based on Langmore [2] and Miller et al. [3] as follows: (1) Observation of anatomical structures with (a) secretion management and (b) sensory testing, (2) direct assessment of swallowing, (3) compensatory strategies, and (4) sensory testing (if tested at this point).
Results
Search Results
A total of 115 records were identified through database search. After screening for inclusion and exclusion criteria, 22 full texts were included in the analysis (Fig. 1).
PRISMA flow diagram of the reviewing process [42]
Risk of Bias and Quality Assessment
All included studies were retrospective or prospective cross-sectional studies, including pilot studies and case series. No study achieved a good rating using the NIH quality assessment tool. Sixteen studies were rated fair and six poor (Table 3). Overall, substantial bias is to be expected because of the lack of methodological quality of the studies and a high number of missing parameters. Particularly critical was the description and definition of valid outcome parameters, especially considering the use of sound statistical methods such as justification of sample sizes and confounding variables.
Study Population
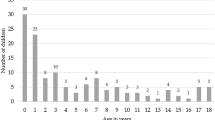
The 22 included studies reported on 1547 children aged 0–18 years in total. The sample size per study ranged from five to 568 children. Two samples included a small number of young adults up to 20 [10] and up to 24 years [11]. The average age of the entire population could not be calculated due to missing values in some of the studies.
Nine studies exclusively focused on infants under 1 year of age [12,13,14,15,16,17,18,19,20]; five samples consisted of children younger than 12 months up to 3 years [21,22,23,24,25]; one study focused on children between 4 and 8 years [26], and a wide age distribution including infants, children and adolescents could be found in seven studies [10, 11, 27,28,29,30] (Table 4).
The overall gender distribution could be calculated for a sample of 18 studies. Among 855 children, an average of 39% were girls. Four studies [13, 15, 25, 28] including Hartnick et al. with the biggest sample size did not report on gender, so the distribution remains unclear for the remaining 682 children.
Further analysis of the samples showed that three studies included only infants from the neonatal intensive care unit (NICU) [12, 14, 18], one focused on mechanically ventilated children [23], and two had a surgical focus [13, 25]. Six studies related to a single diagnosis or symptom: Congenital Zika Syndrome [22], isolated Pierre-Robin sequence [15], laryngomalacia [16] and gastroesophageal reflux disease (GERD) [17], cerebral palsy [26], and prematurity [19]. The remaining 10 samples varied widely in terms of principal diagnosis [10, 11, 20, 21, 24, 27,28,29,30,31] (Table 4).
Implementation Protocols
FEES examinations were usually performed by a (pediatric) otolaryngologist and attended by one or two speech-language pathologists (SLP) or occupational therapists (OT) and a nurse. Two studies reported the performance of FEES by an SLP [12, 18], one by a pediatric neurologist [27], and one by a pediatrician [19]. Five authors did not provide information on the specialization of the person performing FEES [13, 15, 17, 25, 31] (Table 6).
The positioning of the children during endoscope insertion was most frequently reported as upright, as upright as possible, or semi-reclined with stabilization of the head. When breastfed infants were examined, the position preferred by the mothers was adopted after insertion of the endoscope. Five protocols did not include information on the positioning of the child [13, 22, 23, 29, 30].
In some cases, it was explicitly stated that feeding tubes were removed [12, 18] or not removed [16, 23]. Eighteen study protocols did not report on that topic.
Three studies exclusively focused on breastfeeding [12, 16, 20] and seven on bottle-feeding [13,14,15, 18, 19, 23, 25]. Two even provided standardized information on the type of nipple and consistency of milk [14, 18]. Five studies indicated that the boluses evaluated were developmentally appropriate [11, 17, 28, 29, 31]. Standardized boluses were reported in seven protocols, four of which specified the type [21, 24, 27, 32] and three the type and size of the boluses [10, 22, 30] (Table 6).
Equipment
Most examinations were performed with a fiberoptic rhino-laryngoscope. In one study each, a video rhino-laryngoscope and a video bronchoscope were used. The diameters of the endoscopes ranged from 1.9 to 4.1 mm. In two studies, sensory testing via an air impulse channel (FEESST) was also included [25, 31]. Four studies did not report in detail on the endoscope used (Table 6).
In 10 protocols, topical anesthesia was administered as standard. In most cases, lidocaine gel was applied directly to the endoscope. Nine authors reported not using topical anesthesia, and three did not specify. The use of nasal decongestant was reported in two protocols [27, 28].
To calm infants during the uncomfortable insertion of the endoscope, calming techniques were employed in four studies using sucrose solution, non-nutritive sucking or breastfeeding [12, 14, 18, 20].
The use of a thickener was not applicable in the breastfeeding protocols. It was reported for eight studies, four of which indicated the type of thickener as modified corn starch [15, 19, 21, 22] and two as rice cereal [18, 24] (Table 6).
Food dye was reported in fourteen protocols (seven used green, five blue, one yellow in addition to blue, and two did not specify which color). Six studies did not report if they used a food dye and two did not use color but standardized yellow pudding and milk [30] or just white milk [23] (Table 6).
FEES Procedural Steps
The assessment of anatomic structures was included in 14 protocols by mentioning pharyngeal and laryngeal anatomical structures without going into detail. Two of these also named the nasal airway, soft palate, and oropharynx [16, 20]. Ten protocols involving evaluation of secretion management, five protocols testing laryngeal sensitivity at that particular time point [17, 24, 25, 28, 31], and one assessing it as the final step of FEES [11]. In total, sensory testing was performed in six protocols, in one study using the touch method [24] and five using an air pulse [11, 17, 25, 28, 31]. Direct swallowing assessment was the core of all protocols with a single exception. Leder & Karas [10] referred to the standard protocol of Langmore [4] and did not provide further details. Compensatory strategies such as re-positioning, modification of texture, or pacing were part of seven protocols [13, 14, 19, 20, 22, 28] (Table 5).
FEES-based Outcome
In some cases, there was a discrepancy between the FEES-based result advertised in the method and the actual outcome presented in the results. The following section refers to the actual parameters reported in the results.
The parameter combination of secretion pooling and laryngeal sensation was reported in four studies [11, 17, 24, 31], one study reported only secretion pooling [19]. Premature spillage was stated as present or absent in four [21, 22, 24, 31] and delay of swallowing reflex in one study [22]. Penetration was reported in 11, aspiration in 13, and silent aspiration in four studies [10, 16, 22, 27]. Two studies summarized penetration-aspiration [16, 19]. Residues were reported in five result sections [19, 21, 22, 24, 31]. Only one study [22] used Rosenbek’s penetration-aspiration scale (PAS) [33]. No validated scale was used for any other outcome parameter (Table 7).
Thirteen of the studies lacked any information regarding complications or adverse events; in the remaining studies, none were reported.
Subgroup Protocols
FEES protocols for sub-groups could be identified for exclusively breastfed and bottle-fed infants. The breastfed subgroup consisted of 51 participants from three studies (43.5% female) aged 0–10 months [12, 16, 20] (Table 4). An otolaryngologist or an SLP performed the endoscopy. At least one SLP or OT and a nurse assisted. The diameter of the endoscope ranged from 1.9 to 2.7 mm. Lidocaine gel as topical anesthesia was put onto the endoscope according to two protocols [16, 20]. Sucrose solution or breastfeeding in advance was used as a calming strategy in two studies [12, 20]. In two protocols, food dye was applied prior to latching via oral care swab or syringe (Table 6). Mills et al. [16] secured the endoscope with a rubber band before latching. After insertion of the endoscope, all children were positioned in their preferred breastfeeding position. One protocol described only direct assessment of swallowing [12], while the two other studies included the stages observation of anatomical structures and secretion management, direct assessment of swallowing, and compensatory strategies (Table 5). Penetration and aspiration of milk, considered separately, were the endpoints reported in two studies. The third study summarized penetration-aspiration (including silent aspiration) without reporting each item individually [16] (Table 7).
The bottle-fed subgroup was based on seven studies and consisted of 221 children aged 0–26 months [13,14,15, 18, 19, 23, 25]. The gender was reported for 97 children of whom 38.5% were female (Table 4). FEES was performed by an otolaryngologist, an SLP, or a pediatrician, usually assisted by an SLP or OT. Three of the seven studies did not include information on the examiner's profession. The diameter of the endoscope ranged from 2.2–3.6 mm, in case of additional sensory testing via air pulse 4 mm [25]. Mostly, the position of the child was semi-reclined. Three protocols reported on the use of topical anesthesia [13, 19, 25] and two reported on standardized volumes and consistencies [14, 18], whereas the protocol by Kamity et al. [14] included barium due to the simultaneous videofluoroscopy. Five studies used food coloring, three used thickening agents (Table 6). Assessment of anatomic structures, direct evaluation of swallowing, and compensatory strategies was included in three protocols, two of which also included secretion management. [13, 19]. In three protocols, the individual steps of the implementation were not described in detail (Table 5). Aspiration was reported in three studies [14, 23, 25]. Penetration alone was specified in two [14, 25]. One study reported on swallowing dysfunction [13]. One study reported on aspiration risk [15], one summarized penetration-aspiration [19], and one study did not report an exact number of penetration and aspiration in the sample but included it in intra-and interrater correlation [18] (Table 7).
Discussion
The aim of this review was to identify implementation protocols for pediatric FEES described in research studies and to analyze those in detail in terms of procedural steps, equipment, and reported outcomes. It provides important insights into the critical lack of standardization in pediatric FEES protocols and FEES-based studies. It also reflects a rather poor methodological quality of the studies. For this reason, conclusions are limited.
Sample
A wide variation in age, diagnoses, and health conditions of the children evaluated was found both between included studies but also within them. Interestingly, girls accounted for only 39% of the total population (with some missing data). The interesting question here is whether dysphagia in children is more common in one gender. It would therefore be important to also report a gender-differentiated outcome.
Protocols
All implementation protocols were described incompletely and differed in many aspects. As no validated pediatric FEES protocols exist to date, no study could be based on such a protocol. The first comprehensive pediatric protocols were published in 2020 [1, 3], so future studies will be expected to increasingly refer to these. No uniform recommendations for equipment to be used in pediatric FEES have been published to date. A key point to consider here is that very thin, modern chip-on-tip videoscopes are likely to give the best results, while fiberoptic endoscopes allow cost-effective area-wide use. Obtaining full details of the diameter and type of endoscope and other equipment, FEES team, nasal decongestant, topical anesthesia, calming techniques, positioning of the child, and thickening and dyeing of the bolus would enable a better comparison of the examination and the outcome. However, the data from the investigated studies do not allow for comparison.
In summary, the procedural steps proposed by Miller et al. [3] can be reproduced in most protocols. The direct assessment of swallowing is included in all protocols. However, the further descriptions of the study protocols are not detailed enough to allow replication and evaluation.
Few study protocols included standardized bolus amounts and consistencies commonly found in adult protocols. Although children's eating behaviors are distinctly individual, simply stating "developmentally appropriate" is not sufficient for study protocols and should be appropriately specified.
For the reported outcome a similar picture as for procedural steps became apparent. Two problems can be identified here: (i) there are no valid outcome measurement scales for pediatric FEES (ii) the recording of FEES outcome was insufficient for retrospective studies. In a retrospective analysis of pediatric FEES data obtained in our hospital [34], we demonstrated that a large number of missing values were due to incomplete documentation and lack of standardization of protocols.
Since the high rate of silent aspiration in pediatric samples is repeatedly pointed out [35, 36], it would have been very interesting to investigate the factor of silent aspiration for the complete sample. Unfortunately, only four of the 22 studies reported silent aspiration as an outcome parameter.
Overall, no adverse events occurred and FEES was considered safe in all groups, consistent with the findings of Miller and Willging's 25-year experience [1]. However, not all studies consistently reported complications or how many examinations were discontinued or could not be performed at all. This issue is particularly evident in retrospective studies, primarily including cases with a complete FEES and not systematically recording how many FEES could not be performed.
The establishment of specific protocols for breastfed and bottle-fed infants is advisable. Future protocols should take into account that many children, though still breastfed additionally eat puree or are bottle-fed and already receive solid foods.
Limitations
Based on the recently published systematic review by Dharmarathna et al. on quantitative instrumental studies of swallowing in children [6], the methodological quality of pediatric FEES studies was expected to be poor, and the inclusion criteria were expanded accordingly. Meta-analysis of the data was not possible because of a large number of missing data and the range of outcome parameters. In particular, the retrospective studies with large samples had significant deficiencies in the sample description and specification of the data, making further analyses and comparisons impossible. In principle, retrospective analyses of patient data are valuable if they meet certain requirements and systematically provide the necessary data.
Implication for Practice
In practice, the implementation and documentation of pediatric FEES should be standardized and adapted specifically for children and adolescents. Depending on age and nutritional status, fixed procedures and evaluation forms should be available. Since patient groups in practice tend to be heterogeneous, a modular approach may be useful. For infants and young children, a small endoscope, calming techniques, and, where appropriate, a thickening agent and a food dye that can be used safely for infants should be available. For therapy planning and diagnostics, but also to gain more experience, the parameters secretion management and pharyngeal secretion pooling, premature spillage, delay in swallowing reflex, penetration, aspiration (and clearing), silent aspiration, residue, and laryngeal sensation should also be recorded and documented in practice. This is already standard in adults or suggested in recently published recommendations [1, 3].
Implication for Future Research
As a general implication retrospective and prospective studies should focus more on specific age or diagnosis groups. For rare diseases, case numbers should be increased through multicenter collaboration or meta-analysis. For this purpose, FEES protocols must be described in sufficient detail to allow replication. This includes the FEES performing team, technical and other equipment, bolus types and sizes, calming strategies, exact procedural steps, and outcome.
The positioning of the child during insertion of the endoscope and throughout the subsequent examination, as well as the entire setting, should be described and illustrated with a photograph or drawing. Calming techniques such as sucrose solution and non-nutritive sucking, distraction by videos, or consultation with a child life specialist (as suggested by Miller & Willging [1]) should be mentioned.
A systematic report of the outcome is essential. Based on valid scales for adult FEES, the evaluation of all parameters should be recorded in scale form rather than just as present or absent. However, adaptation and validation of those scales for pediatric FEES are still needed: pharyngeal secretion pooling (e.g., Murray secretion scale [37]), premature spillage (e.g., Langmore and colleagues [38]), delayed swallowing reflex (e.g., Warnecke and colleagues [39]) penetration (alone), aspiration and clearing, silent aspiration (e.g., PAS [33]), residue (e.g., Yale Pharyngeal Residue Severity Rating Scale [40]), and laryngeal sensation (e.g., Marian et al. [41]). Preferably, results are also reported for each gender separately. Findings of interest, specific to certain groups should also be reported.
A final important issue for future research concerns compliance and general behavior of children during FEES. Future studies should report whether excessive crying, severe resistance, or refusal to eat or drink occurred during the examination and how this affected the acquisition of meaningful swallowing images. By specifying the average duration of the examination and after what time and how it was possible to calm down the child or not, it would help in future to find out more about the acceptance of the examination (e.g., in certain age groups). In addition, it should be summarized how many examinations had to be prematurely terminated or could not be performed at all. Of course, other reasons for termination of examinations such as choanal stenosis should also be given.
Researchers and practitioners using FEES should always keep in mind that swallowing function can be distorted by strong, sustained crying or discomfort. The starting point for a meaningful study should therefore always be the greatest possible comfort for the children and their parents. Future research must deal with how this comfort can be achieved.
Conclusion
There is currently no pediatric FEES protocol that fully addresses the implementation, equipment, and, most importantly, outcome. Promising approaches are offered by protocols for infants who are breastfed, bottle-fed, or cared for in the neonatal intensive care unit. Even though the included studies did not exhibit good methodological quality and lack of data did not allow for direct comparison, this systematic review provides an important foundation for future pediatric FEES studies. An invaluable basis for this is provided by the empirical values and innovative ideas of the authors and researchers of the included studies.
References
Miller CK, Willging JP. Fiberoptic endoscopic evaluation of swallowing in infants and children: protocol, safety, and clinical efficacy: 25 years of experience. Ann Otol Rhinol Laryngol. 2020;129(5):469–81. https://doi.org/10.1177/0003489419893720.
Langmore SE. History of fiberoptic endoscopic evaluation of swallowing for evaluation and management of pharyngeal dysphagia: changes over the years. Dysphagia. 2017;32(1):27–38. https://doi.org/10.1007/s00455-016-9775-x.
Miller CK, Schroeder JW Jr, Langmore S. Fiberoptic endoscopic evaluation of swallowing across the age spectrum. Am J Speech Lang Pathol. 2020;29(2s):967–78. https://doi.org/10.1044/2019_ajslp-19-00072.
Langmore SE, Schatz K, Olsen N. Fiberoptic endoscopic examination of swallowing safety: a new procedure. Dysphagia. 1988;2(4):216–9. https://doi.org/10.1007/BF02414429.
Prikladnicki A, Santana MG, Cardoso MC. Protocols and assessment procedures in fiberoptic endoscopic evaluation of swallowing: an updated systematic review. Braz J Otorhinolaryngol. 2021. https://doi.org/10.1016/j.bjorl.2021.03.002.
Dharmarathna I, Miles A, Allen J. Twenty years of quantitative instrumental measures of swallowing in children: a systematic review. Eur J Pediatr. 2020;179(2):203–23. https://doi.org/10.1007/s00431-019-03546-x.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, Group P-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. https://doi.org/10.1186/2046-4053-4-1.
NIH National Heart LaBIN. Study quality assessment tools: quality assessment tool for observational cohort and cross-sectional studies: U.S. Department of Health & Human Services. Available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed: 1 Oct 2021.
Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, Hartmann-Boyce J, Ryan R, Shepperd S, Thomas J, Welch V, Thomson H. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368:l6890. https://doi.org/10.1136/bmj.l6890.
Leder SB, Karas DE. Fiberoptic endoscopic evaluation of swallowing in the pediatric population. Laryngoscope. 2000;110(7):1132–6. https://doi.org/10.1097/00005537-200007000-00012.
Link DT, Willging JP, Miller CK, Cotton RT, Rudolph CD. Pediatric laryngopharyngeal sensory testing during flexible endoscopic evaluation of swallowing: feasible and correlative. Ann Otol Rhinol Laryngol. 2000;109(10 Pt 1):899–905. https://doi.org/10.1177/000348940010901002.
Armstrong ES, Reynolds J, Sturdivant C, Carroll S, Suterwala MS. Assessing swallowing of the breastfeeding NICU infant using fiberoptic endoscopic evaluation of swallowing: a feasibility study. Adv Neonatal Care. 2020;20(3):244–50. https://doi.org/10.1097/ANC.0000000000000696.
Averin K, Uzark K, Beekman RH 3rd, Willging JP, Pratt J, Manning PB. Postoperative assessment of laryngopharyngeal dysfunction in neonates after Norwood operation. Ann Thorac Surg. 2012;94(4):1257–61. https://doi.org/10.1016/j.athoracsur.2012.01.009.
Kamity R, Ferrara L, Dumpa V, Reynolds J, Islam S, Hanna N. Simultaneous videofluoroscopy and endoscopy for dysphagia evaluation in preterm infants-a pilot study. Front Pediatr. 2020;8:537. https://doi.org/10.3389/fped.2020.00537.
Marques IL, Prado-Oliveira R, Leirião VH, Jorge JC, de Souza L. Clinical and fiberoptic endoscopic evaluation of swallowing in Robin sequence treated with nasopharyngeal intubation: the importance of feeding facilitating techniques. Cleft Palate Craniofac J. 2010;47(5):523–9. https://doi.org/10.1597/09-002.
Mills N, Keesing M, Geddes D, Mirjalili SA. Flexible endoscopic evaluation of swallowing in breastfeeding infants with laryngomalacia: observed clinical and endoscopic changes with alteration of infant positioning at the breast. Ann Otol Rhinol Laryngol. 2020. https://doi.org/10.1177/0003489420965636.
Suskind DL, Thompson DM, Gulati M, Huddleston P, Liu DC, Baroody FM. Improved infant swallowing after gastroesophageal reflux disease treatment: a function of improved laryngeal sensation? Laryngoscope. 2006;116(8):1397–403. https://doi.org/10.1097/01.mlg.0000225942.33102.9b.
Suterwala MS, Reynolds J, Carroll S, Sturdivant C, Armstrong ES. Using fiberoptic endoscopic evaluation of swallowing to detect laryngeal penetration and aspiration in infants in the neonatal intensive care unit. J Perinatol. 2017;37(4):404–8. https://doi.org/10.1038/jp.2016.239.
Vetter-Laracy S, Osona B, Roca A, Pena-Zarza JA, Gil JA, Figuerola J. Neonatal swallowing assessment using fiberoptic endoscopic evaluation of swallowing (FEES). Pediatr Pulmonol. 2018;53(4):437–42. https://doi.org/10.1002/ppul.23946.
Willette S, Molinaro LH, Thompson DM, Schroeder JW Jr. Fiberoptic examination of swallowing in the breastfeeding infant. Laryngoscope. 2016;126(7):1681–6. https://doi.org/10.1002/lary.25641.
da Silva AP, Lubianca Neto JF, Santoro PP. Comparison between videofluoroscopy and endoscopic evaluation of swallowing for the diagnosis of dysphagia in children. Otolaryngol Head Neck Surg. 2010;143(2):204–9. https://doi.org/10.1016/j.otohns.2010.03.027.
Leal MC, van der Linden V, Bezerra TP, de Valois L, Borges ACG, Antunes MMC, Brandt KG, Moura CX, Rodrigues LC, Ximenes CR. Characteristics of dysphagia in infants with microcephaly caused by congenital zika virus infection, Brazil, 2015. Emerg Infect Dis. 2017;23(8):1253–9. https://doi.org/10.3201/eid2308.170354.
Leder SB, Baker KE, Goodman TR. Dysphagia testing and aspiration status in medically stable infants requiring mechanical ventilation via tracheotomy. Pediatr Crit Care Med. 2010;11(4):484–7. https://doi.org/10.1097/PCC.0b013e3181ceae50.
Pavithran J, Puthiyottil IV, Kumar M, Nikitha AV, Vidyadharan S, Bhaskaran R, Chandrababu Jaya A, Thankappan K, Subramania I, Sundaram KR. Exploring the utility of fibreoptic endoscopic evaluation of swallowing in young children—a comparison with videofluoroscopy. Int J Pediatr Otorhinolaryngol. 2020;138:110339. https://doi.org/10.1016/j.ijporl.2020.110339.
Richter GT, Wootten CT, Rutter MJ, Thompson DM. Impact of supraglottoplasty on aspiration in severe laryngomalacia. Ann Otol Rhinol Laryngol. 2009;118(4):259–66. https://doi.org/10.1177/000348940911800404.
Umay E, Gündoğdu İ, Öztürk EA. Reliability and validity of the pediatric feeding and swallowing disorders family impact scale for Turkish children with cerebral palsy by endoscopic evaluation. Turk J Pediatr. 2019;61(5):741–8. https://doi.org/10.24953/turkjped.2019.05.013.
Beer S, Hartlieb T, Müller A, Granel M, Staudt M. Aspiration in children and adolescents with neurogenic dysphagia: comparison of clinical judgment and fiberoptic endoscopic evaluation of swallowing. Neuropediatrics. 2014;45(6):402–5. https://doi.org/10.1055/s-0034-1387814.
Hartnick CJ, Hartley BE, Miller C, Willging JP. Pediatric fiberoptic endoscopic evaluation of swallowing. Ann Otol Rhinol Laryngol. 2000;109(11):996–9. https://doi.org/10.1177/000348940010901102.
Sitton M, Arvedson J, Visotcky A, Braun N, Kerschner J, Tarima S, Brown D. Fiberoptic endoscopic evaluation of swallowing in children: feeding outcomes related to diagnostic groups and endoscopic findings. Int J Pediatr Otorhinolaryngol. 2011;75(8):1024–31. https://doi.org/10.1016/j.ijporl.2011.05.010.
Suiter DM, Leder SB, Karas DE. The 3-ounce (90-cc) water swallow challenge: a screening test for children with suspected oropharyngeal dysphagia. Otolaryngol Head Neck Surg. 2009;140(2):187–90. https://doi.org/10.1016/j.otohns.2008.11.016.
Ulualp S, Brown A, Sanghavi R, Rivera-Sanchez Y. Assessment of laryngopharyngeal sensation in children with dysphagia. Laryngoscope. 2013;123(9):2291–5. https://doi.org/10.1002/lary.24024.
Umay E, Gundogdu I, Ozturk EA. Reliability and validity of the pediatric feeding and swallowing disorders family impact scale for Turkish children with cerebral palsy by endoscopic evaluation. Turk J Pediatr. 2019;61(5):741–8. https://doi.org/10.24953/turkjped.2019.05.013.
Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11(2):93–8. https://doi.org/10.1007/BF00417897.
Zang J, Nienstedt JC, Koseki JC, Niessen A, Flugel T, Kim SH, Pflug C. Pediatric flexible endoscopic evaluation of swallowing: critical analysis of implementation and future perspectives. Dysphagia. 2021. https://doi.org/10.1007/s00455-021-10312-5.
Garand KLF, McCullough G, Crary M, Arvedson JC, Dodrill P. Assessment across the life span: the clinical swallow evaluation. Am J Speech Lang Pathol. 2020;29(2S):919–33. https://doi.org/10.1044/2020_AJSLP-19-00063.
Arvedson JC, Lefton-Greif MA. Instrumental assessment of pediatric dysphagia. Semin Speech Lang. 2017;38(2):135–46. https://doi.org/10.1055/s-0037-1599111.
Murray J, Langmore SE, Ginsberg S, Dostie A. The significance of accumulated oropharyngeal secretions and swallowing frequency in predicting aspiration. Dysphagia. 1996;11(2):99–103. https://doi.org/10.1007/BF00417898.
Langmore SE, Olney RK, Lomen-Hoerth C, Miller BL. Dysphagia in patients with frontotemporal lobar dementia. Arch Neurol. 2007;64(1):58–62. https://doi.org/10.1001/archneur.64.1.58.
Warnecke T, Oelenberg S, Teismann I, Hamacher C, Lohmann H, Ringelstein EB, Dziewas R. Endoscopic characteristics and levodopa responsiveness of swallowing function in progressive supranuclear palsy. Mov Disord. 2010;25(9):1239–45. https://doi.org/10.1002/mds.23060.
Neubauer PD, Rademaker AW, Leder SB. The yale pharyngeal residue severity rating scale: an anatomically defined and image-based tool. Dysphagia. 2015;30(5):521–8. https://doi.org/10.1007/s00455-015-9631-4.
Marian T, Schroder JB, Muhle P, Claus I, Riecker A, Warnecke T, Suntrup-Krueger S, Dziewas R. Pharyngolaryngeal sensory deficits in patients with middle cerebral artery infarction: lateralization and relation to overall dysphagia severity. Cerebrovasc Dis Extra. 2017;7(3):130–9. https://doi.org/10.1159/000479483.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med. 2009;3(3):e123-130.
Warnecke, T, Teismann, I, Oelenberg, S, Hamacher, C, Ringelstein, EB, Schäbitz, WR, & Dziewas, R. Towards a basic endoscopic evaluation of swallowing in acute stroke–identification of salient findings by the inexperienced examiner. BMC Med. Educ, 2009;9(1): 1–7. https://doi.org/10.1186/1472-6920-9-13
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
JZ drafted the manuscript. SK, TF, SHK, and J-CK contributed to the development of the selection criteria, the risk of bias assessment strategy, and data extraction criteria. JZ and CP developed the search strategy. JCN and CP provided expertise on FEES. AN corrected the manuscript and provided expertise on dysphagia terminology. All authors read, provided feedback, and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest. All authors have reviewed and approved the contents of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zang, J., Kiehn, S., Flügel, T. et al. Implementation of Pediatric Flexible-Endoscopic Evaluation of Swallowing: A Systematic Review and Recommendations for Future Research. Dysphagia 37, 1822–1838 (2022). https://doi.org/10.1007/s00455-022-10446-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-022-10446-0