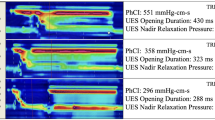
Abstract
Several physiological metrics can be derived from pharyngeal high-resolution impedance manometry (HRPM), but their clinical relevance has not been well established. We investigated the diagnostic performance of these metrics in relation to videofluoroscopic (VFS) assessment of aspiration and residue in patients with oropharyngeal dysphagia. We analyzed 263 swallows from 72 adult patients (22–91 years) with diverse medical conditions. Metrics of contractility, upper esophageal sphincter (UES) opening and relaxation, flow timing, intrabolus distension pressure, and a global Swallow Risk Index (SRI) were derived from pressure-impedance recordings using pressure-flow analysis. VFS data were independently scored for airway invasion and pharyngeal residue using the Penetration-Aspiration Scale and the Normalized Residue Ratio Scale, respectively. We performed multivariate logistic regression analyses to determine the relationship of HRPM metrics with radiological outcomes and receiver-operating characteristic (ROC) analysis to evaluate their diagnostic accuracy. We identified aspiration in 25% and pharyngeal residue in 84% of the swallows. Aspiration was independently associated with hypopharyngeal peak pressure < 65 mmHg (HypoPeakP) [adjusted odds ratio (OR) 5.27; 95% Confidence Interval (CI) (0.99–28.1); p = 0.051], SRI > 15 [OR 4.37; 95% CI (1.87–10.2); p < 0.001] and proximal esophageal contractile integral (PCI) < 55 mmHg·cm·s [OR 2.30; 95% CI (1.07–4.96); p = 0.034]. Pyriform sinus residue was independently predicted by HypoPeakP < 65 mmHg [OR 7.32; 95% CI (1.93–27.7); p = 0.003], UES integrated relaxation pressure (UES-IRP) > 3 mmHg [OR 2.96; 95% CI (1.49–5.88); p = 0.002], and SRI > 15 [OR 2.17; 95% CI (1.04–4.51); p = 0.039]. Area under ROC curve (AUC) values for individual HRPM metrics ranged from 0.59 to 0.74. Optimal cut-off values were identified. This study demonstrates the diagnostic value of certain proposed and adjunct HRPM metrics for identifying signs of unsafe and inefficient bolus transport in patients with oropharyngeal dysphagia.



Similar content being viewed by others
Abbreviations
- BPT:
-
Hypopharyngeal bolus presence time
- DCL:
-
Hypopharyngeal distension to contraction latency
- HCI:
-
Hypopharyngeal contractile integral
- HRPM:
-
High-resolution pharyngeal manometry
- HypoPeakP:
-
Hypopharyngeal mean peak pressure
- IBP:
-
Hypopharyngeal intrabolus pressure
- IRP:
-
Integrated relaxation pressure
- MaxAd:
-
Maximum admittance
- MBSImP:
-
Modified Barium Swallow Impairment Profile
- MCI:
-
Mesopharyngeal contractile integral
- NRRSp:
-
Normalized residue ratio scale (pyriform sinus residue)
- NRRSv:
-
Normalized residue ratio scale (vallecular residue)
- OPD:
-
Oropharyngeal dysphagia
- PAS:
-
Penetration-Aspiration Scale
- PCI:
-
Proximal esophageal contractile integral
- PFA:
-
Pressure-flow analysis
- PhCI:
-
Pharyngeal contractile integral
- RT:
-
Relaxation time
- SRI:
-
Swallow risk index
- UES:
-
Upper esophageal sphincter
- VCI:
-
Velopharyngeal contractile integral
- VFS:
-
Videofluoroscopy
References
Rommel N, Hamdy S. Oropharyngeal dysphagia: manifestations and diagnosis. Nat Rev Gastroenterol Hepatol. 2016;13:49–59. https://doi.org/10.1038/nrgastro.2015.199.
Cabre M, Serra-Prat M, Force L, Almirall J, Palomera E, Clave P. Oropharyngeal dysphagia is a risk factor for readmission for pneumonia in the very elderly persons: observational prospective study. J Gerontol A. 2014;69(3):330–7. https://doi.org/10.1093/gerona/glt099.
Ekberg O, Hamdy S, Woisard V, Wuttge-Hannig A, Ortega P. Social and psychological burden of dysphagia: its impact on diagnosis and treatment. Dysphagia. 2002;17(2):139–46. https://doi.org/10.1007/s00455-001-0113-5.
Patel DA, Krishnaswami S, Steger E, Conover E, Vaezi MF, Ciucci MR, et al. Economic and survival burden of dysphagia among inpatients in the United States. Dis Esophagus. 2017. https://doi.org/10.1093/dote/dox131.
Smithard DG. Dysphagia: a geriatric giant? Med Clin Rev. 2016;2(15):1–7. https://doi.org/10.21767/2471-299X.1000014.
Clavé P, Shaker R. Dysphagiass: current reality and scope. Nat Rev Gastroenterol Hepatol. 2015;12(5):259–70. https://doi.org/10.1038/nrgastro.2015.49.
Baijens L, Barikroo A, Pilz W. Intrarater and interrater reliability for measurements in videofluoroscopy of swallowing. Eur J Radiol. 2013;82(10):1683–95. https://doi.org/10.1016/j.ejrad.2013.05.009.
Mccullough GH, Wertz RT, Rosenbek JC, Mills RH, Webb WG, Ross KB. Inter- and intrajudge reliability for videofluoroscopic swallowing evaluation measures. Dysphagia. 2001;16:110–8. https://doi.org/10.1007/s004550010004.
Swan K, Cordier R, Brown T, Speyer R. Psychometric properties of visuoperceptual measures of videofluoroscopic and fibre-endoscopic evaluations of swallowing : a systematic review. Dysphagia. 2018. https://doi.org/10.1007/s00455-018-9918-3.
Cock C, Omari T. Diagnosis of swallowing disorders: how we interpret pharyngeal manometry. Curr Gastroenterol Rep. 2017;19(11):1–14. https://doi.org/10.1007/s11894-017-0552-2.
Huckabee ML, Macrae P, Lamvik K. Expanding instrumental options for dysphagia diagnosis and research: ultrasound and manometry. Folia Phoniatr Logop. 2015;67:269–84. https://doi.org/10.1159/000444636.
Cook IJ. Combined pharyngeal impedance-manometry: has it finally come of age? Clin Gastroenterol Hepatol. 2011;9(10):813–5. https://doi.org/10.1016/j.cgh.2011.06.021.
Knigge MA, Marvin S, Thibeault SL. Safety and tolerability of pharyngeal high-resolution manometry. Am J Speech-Language Pathol. 2018. https://doi.org/10.1044/2018_AJSLP-18-0039.
Mielens JD, Hoffman MR, Ciucci M, Jiang JJ, Mcculloch TM. Automated analysis of pharyngeal pressure data obtained with high-resolution manometry. Dysphagia. 2011;26:3–12. https://doi.org/10.1007/s00455-010-9320-2.
Nativ-Zeltzer N, Logemann JA, Zecker SG, Kahrilas PJ. Pressure topography metrics for high-resolution pharyngeal-esophageal manofluorography—a normative study of younger and older adults. Neurogastroenterol Motil. 2016;28:721–31. https://doi.org/10.1111/nmo.12769.
Omari TI, Ciucci M, Gozdzikowska K, Hernández E, Hutcheson K, Jones C, et al. High-resolution pharyngeal manometry and impedance: protocols and metrics—recommendations of a High-Resolution Pharyngeal Manometry International Working Group. Dysphagia. 2019. https://doi.org/10.1007/s00455-019-10023-y.
Omari TI, Dejaeger E, Van BD, Goeleven A, Davidson GP, Dent J, et al. A method to objectively assess swallow function in adults with suspected aspiration. Gastroenterology. 2011;140(5):1454–63. https://doi.org/10.1053/j.gastro.2011.02.051.
Omari TI, Dejaeger E, Van BD, Goeleven A, De CP, Smet MH, et al. A novel method for the nonradiological assessment of ineffective swallowing. Am J Gastroenterol. 2011;106(10):1796–802. https://doi.org/10.1038/ajg.2011.143.
Ferris L, Doeltgen S, Cock C, Rommel N, Schar M, Carrion S, et al. Modulation of pharyngeal swallowing by bolus volume and viscosity. Am J Physiol Gastrointest Liver Physiol. 2020. https://doi.org/10.1152/ajpgi.00270.2020.
Omari TI. Swallow Gateway for High Resolution Pharyngeal & Esophageal Manometry (Version 4) [Internet]. 2019 [cited 2019 Apr 29]. p. 1–47. Available from: https://www.swallowgateway.com/Swallow Gateway Instruction Manual_Version 4 (April 2019).pdf
Cichero JAY, Lam P, Steele CM, Hanson B, Chen J, Dantas RO, et al. Development of international terminology and definitions for texture-modified foods and thickened fluids used in dysphagia management: the IDDSI Framework. Dysphagia. 2017;32(2):293–214. https://doi.org/10.1007/s00455-016-9758-y.
Singendonk M, Cock C, Bieckmann L, Szczesniak M, Benninga M, Omari T. Reliability of an online analysis platform for pharyngeal high-resolution impedance manometry recordings. Speech Lang Hear. 2018. https://doi.org/10.1080/2050571X.2018.1535564.
Steele CM, Peladeau-Pigeon M, Barbon CA, Guida BT, MacDonald AN, Nascimento WV, et al. Reference values for healthy swallowing across the range from thin to extremely thick liquids. J Speech Lang Hear Res. 2019. https://doi.org/10.1044/2019_JSLHR-S-18-0448.
Martin-Harris B, Brodsky MB, Michel Y, Castell DO, Schleicher M, Sandidge J, et al. MBS measurement tool for swallow impairment–MBSImp: establishing a standard. Dysphagia. 2008;23(4):392–405. https://doi.org/10.1007/s00455-008-9185-9.
Rosenbek JC, Robbins JA, Roecker PDEB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;98(11):93–8. https://doi.org/10.1007/BF00417897.
Steele CM, Grace-Martin K. Reflections on clinical and statistical use of the Penetration-Aspiration Scale. Dysphagia. 2017;32(5):601–16. https://doi.org/10.1007/s00455-017-9809-z.
Rommel N, Borgers C, Van BD, Goeleven A, Dejaeger E, Omari TI. Bolus Residue Scale: an easy-to-use and reliable videofluoroscopic analysis tool to score bolus residue in patients with dysphagia. Int J Otolaryngol. 2015. https://doi.org/10.1155/2015/780197.
Pearson WG, Sonja J, Smith ZM, Steele CM. Image-based measurement of post-swallow residue: the Normalized Residue Ratio Scale. Dysphagia. 2013;28:167–77. https://doi.org/10.1007/s00455-012-9426-9.
Steele CM. NRRS Rating Instructions. 2015 [cited 2019 Apr 2]. http://steeleswallowinglab.ca/srrl/wp-content/uploads/2015/03/NRRS_Rating_Instructions.pdf
Hosmer DW, Lemeshow S. Applied logistic regression. New York: Wiley; 1989.
Unal I. Defining an optimal cut-point value in ROC analysis: an alternative approach. Comput Math Methods Med. 2017;2017:1–14. https://doi.org/10.1155/2017/3762651.
O’Rourke AK, Humphries K, Lazar A, Martin-Harris B. The pharyngeal contractile integral is a useful indicator of pharyngeal swallowing impairment. Neurogastroenterol Motil. 2017;29:1–7. https://doi.org/10.1111/nmo.13144.
Kern MK, Balasubramanian G, Sanvanson P, Agrawal D, Wuerl A, Shaker R. Pharyngeal peristaltic pressure variability, operational range, and functional reserve. Am J Physiol Gastrointest Liver Physiol. 2019;312:516–25. https://doi.org/10.1152/ajpgi.00382.2016.
Balasubramanian G, Sharma T, Kern M, Mei L, Sanvanson P, Shaker R. Characterization of pharyngeal peristaltic pressure variability during volitional swallowing in healthy individuals. Neurogastroenterol Motil. 2017;29(11):1–8. https://doi.org/10.1111/nmo.13119.
Prabhakar V, Hasenstab KA, Osborn E, Wei L, Jadcherla SR. Pharyngeal contractile and regulatory characteristics are distinct during nutritive oral stimulus in preterm-born infants: Implications for clinical and research applications. Neurogastroenterol Motil. 2019. https://doi.org/10.1111/nmo.13650.
Jadcherla SR, Prabhakar V, Hasenstab KA, Nawaz S, Das J, Kern M. Defining pharyngeal contractile integral during high-resolution manometry in neonates: a neuromotor marker of pharyngeal vigor. Pediatr Res. 2018;84:341–7. https://doi.org/10.1038/s41390-018-0097-6.
Shaker R, Sanvanson P, Balasubramanian G, Kern M, Wuerl A, Hyngstrom A. Effects of laryngeal restriction on pharyngeal peristalsis and biomechanics: clinical implications. Am J Physiol Gastrointest Liver Physiol. 2016;310:1036–43. https://doi.org/10.1152/ajpgi.00010.2016.
Regan J. Impact of sensory stimulation on pharyngo-esophageal swallowing biomechanics in adults with dysphagia: a high-resolution manometry study. Dysphagia. 2020;35(5):825–33. https://doi.org/10.1007/s00455-019-10088-9.
Agrawal D, Kern M, Edeani F, Balasubramanian G, Hyngstrom A, Sanvanson P, et al. Swallow strength training exercise for elderly: a health maintenance need. Neurogastroenterol Motil. 2017;2018:1–9. https://doi.org/10.1111/nmo.13382.
Park C, Lee Y, Yi Y, Lee J, Park JH. Ability of high-resolution manometry to determine feeding method and to predict aspiration pneumonia in patients with dysphagia. Am J Gastroenterol [Internet]. 2017;112(7):1074–83. https://doi.org/10.1038/ajg.2017.81.
Park D, Oh Y, Ryu S. Findings of abnormal videofluoroscopic swallowing study identified by high-resolution manometry parameters. Arch Phys Med Rehabil. 2016;97(3):421–8. https://doi.org/10.1016/j.apmr.2015.10.084.
Perry JL. Anatomy and physiology of the velopharyngeal mechanism. Semin Speech Lang. 2011;1(212):83–93. https://doi.org/10.1055/s-0031-1277712.
Pearson WG, Hindson DF, Langmore SE, Zumwalt AC. Evaluating swallowing muscles essential for hyolaryngeal elevation by using muscle functional magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2013;85(3):735–40. https://doi.org/10.1016/j.ijrobp.2012.07.2370.
Doeltgen SH, Ong E, Scholten I, Cock C, Omari T. Biomechanical quantification of Mendelsohn maneuver and effortful swallowing on pharyngoesophageal function. Otolaryngology. 2017;157(5):816–23. https://doi.org/10.1177/0194599817708173.
Peng L, Patel A, Kushnir V, Gyawali CP. Assessment of upper esophageal sphincter function on high-resolution manometry: identification of predictors of globus symptoms. J Clin Gastroenterol. 2015;49(2):95–100. https://doi.org/10.1097/MCG.0000000000000078.
Chen S, Liang M, Zhang M, Tan N, Lin Y, Cao P. A study of proximal esophageal baseline impedance in identifying and predicting laryngopharyngeal reflux. J Gastroenterol Hepatol. 2020. https://doi.org/10.1111/jgh.14998.
Pouderoux P, Kahrilas PJ. Function of upper esophageal sphincter during swallowing: the grabbing effect. Am J Physiol Gastrointest Liver Physiol. 1997;272:G1057–63. https://doi.org/10.1152/ajpgi.1997.272.5.G1057.
Edeani FO, Kern M, Ulualp K, et al. Variables influencing manometric parameters of deglutitive and non-deglutitive upper esophageal sphincter: a study of 89 asymptomatic participants. Neurogastroenterol Motil. 2021. https://doi.org/10.1111/nmo.14175.
Acknowledgements
Part of this study was completed as a master’s dissertation to fulfill the requirements for the degree of Master of Deglutology at the Faculty of Medicine, KULeuven. This dissertation was written by the first author (HB), who received a scholarship grant from the Department of Science and Technology—Science Education Institute (DOST-SEI) of the Republic of the Philippines to study in Leuven, Belgium.
Funding
No funds, grants, or other support was received for conducting this study.
Author information
Authors and Affiliations
Contributions
HHGB—conceptualization, data curation, formal analysis, investigation, methodology, project administration, validation, visualization, writing—original draft, writing—review & editing. NP—conceptualization, formal analysis, investigation, writing—original draft. AG—investigation, resources. JT—resources—review & editing. TO—conceptualization, software, supervision, writing—review & editing. NR—conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing—original draft, writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
Nathalie Rommel and Taher Omari hold a patent on the AIM software used for pressure-flow analysis. The other authors have no conflicts of interest to declare in relation to this study.
Ethical Approval
The study protocol was approved by the Research Ethics Committee of University Hospitals Leuven, Belgium.
Informed Consent
All subjects provided written and verbal consent to allow their anonymized clinical data to be used for research purposes.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bayona, H.H.G., Pizzorni, N., Tack, J. et al. Accuracy of High-Resolution Pharyngeal Manometry Metrics for Predicting Aspiration and Residue in Oropharyngeal Dysphagia Patients with Poor Pharyngeal Contractility. Dysphagia 37, 1560–1575 (2022). https://doi.org/10.1007/s00455-022-10417-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-022-10417-5