Abstract
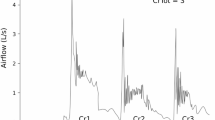
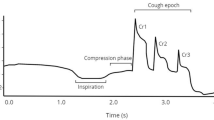
Cough is an important airway protective behavior responsible for ejecting material from the airway to prevent pneumonia, a leading cause of death in older adults and individuals with Parkinson’s disease (PD). Variability of motor performance for both spinal and bulbar functions has been documented; however, there are no studies examining variability of cough motor control in PD and healthy controls. The present study examined the effects of age and PD on variability of voluntary cough performance. Twenty-five healthy younger adults (HYA), 26 healthy older adults (HOA), and 16 participants with PD completed three trials of sequential voluntary cough with spirometry. Coefficients of variation were used to examine variability between groups. Increased variability of cough expired volume (p = 0.012) and inspiratory volume (p = 0.006) was appreciated in HOAs compared to HYAs. Participants with PD demonstrated increased variability of cough expired volume (p = 0.029), peak expiratory flow rise time (p = 0.016), and cough volume acceleration (p = 0.034) compared to HOAs. Though participants with PD descriptively demonstrated increased peak expiratory flow rate compared to HOAs, this finding was statistically nonsignificant after adjusting for multiple comparisons (p = 0.072). This study identified that variability in cough airflow increases in healthy aging and Parkinson’s disease. These motor control impairments may be attributed to age and disease-related sensorimotor changes in the peripheral and central nervous system. Future research will be necessary to examine the relationship between inconsistent cough motor output, airway invasion, and aspiration pneumonia in PD.


Similar content being viewed by others
References
Hegland KW, Okun MS, Troche MS. Sequential voluntary cough and aspiration or aspiration risk in Parkinson’s disease. Lung. 2014;192(4):601–8.
Pitts T, Troche M, Mann G, Rosenbek J, Okun MS, Sapienza C. Using voluntary cough to detect penetration and aspiration during oropharyngeal swallowing in patients with Parkinson disease. Chest. 2010;138(6):1426–31.
Bianchi C, Baiardi P, Khirani S, Cantarella G. Cough peak flow as a predictor of pulmonary morbidity in patients with dysphagia. Am J Phys Med Rehabil. 2012;91(9):783–8.
Tunik E, Houk JC, Grafton ST. Basal ganglia contribution to the initiation of corrective submovements. NeuroImage. 2009;47(4):1757–66.
Houk JC, Bastianen C, Fansler D, Fishbach A, Fraser D, Reber PJ, et al. Action selection and refinement in subcortical loops through basal ganglia and cerebellum. Phil Trans R Soc B. 2007;362(1485):1573–83.
Dickson DW. Neuropathology of Parkinson disease. Parkinsonism RelatDisord. 2018;46:30–3.
Reeves S, Bench C, Howard R. Ageing and the nigrostriatal dopaminergic system. Int J Geriat Psychiatry. 2002;17(4):359–70.
Baltadjieva R, Giladi N, Gruendlinger L, Peretz C, Hausdorff JM. Marked alterations in the gait timing and rhythmicity of patients with de novo Parkinson’s disease. Eur J Neurosci. 2006;24(6):1815–20.
Callisaya ML, Blizzard L, Schmidt MD, McGinley JL, Srikanth VK. Ageing and gait variability—a population-based study of older people. Age Ageing. 2010;39(2):191–7.
Pradhan S, Scherer R, Matsuoka Y, Kelly VE. Grip force modulation characteristics as a marker for clinical disease progression in individuals with Parkinson disease: case-control study. Phys Ther. 2015;95(3):369–79.
Vaillancourt DE, Slifkin AB, Newell KM. Inter-digit individuation and force variability in the precision grip of young, elderly, and Parkinson’s disease participants. Mot Control. 2002;6(2):113–28.
Arias P, Robles-García V, Espinosa N, Corral Y, Cudeiro J. Validity of the finger tapping test in Parkinson’s disease, elderly and young healthy subjects: is there a role for central fatigue? Clin Neurophysiol. 2012;123(10):2034–41.
Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001;82(8):1050–6.
Streicher M, Wirth R, Schindler K, Sieber CC, Hiesmayr M, Volkert D. Dysphagia in nursing homes—results from the NutritionDay Project. J Am Med Directors Assoc. 2018;19(2):141–147.e2.
Serra-Prat M, Palomera M, Gomez C, Sar-Shalom D, Saiz A, Montoya JG, et al. Oropharyngeal dysphagia as a risk factor for malnutrition and lower respiratory tract infection in independently living older persons: a population-based prospective study. Age Ageing. 2012;41(3):376–81.
Langmore SE, Terpenning MS, Schork A, Chen Y, Murray JT, Lopatin D, et al. Predictors of aspiration pneumonia: how important is dysphagia? Dysphagia. 1998;13(2):69–81.
Molfenter SM, Steele CM. Physiological variability in the deglutition literature: hyoid and laryngeal kinematics. Dysphagia. 2011;26(1):67–74.
Molfenter SM, Steele CM. Temporal variability in the deglutition literature. Dysphagia. 2012;27(2):162–77.
Jones CA, Ciucci MR. Multimodal swallowing evaluation with high-resolution manometry reveals subtle swallowing changes in early and mid-stage Parkinson disease. JPD. 2016;6(1):197–208.
Akbar U, Dham B, He Y, Hack N, Wu S, Troche M, et al. Incidence and mortality trends of aspiration pneumonia in Parkinson’s disease in the United States, 1979–2010. Parkinsonism RelatDisord. 2015;21(9):1082–6.
Brandimore AE, Hegland KW, Okun MS, Davenport PW, Troche MS. Voluntary upregulation of reflex cough is possible in healthy older adults and Parkinson’s disease. J ApplPhysiol. 2017;123(1):19–26.
Brandimore AE, Troche MS, Huber JE, Hegland KW. Respiratory kinematic and airflow differences between reflex and voluntary cough in healthy young adults. Front Physiol. 2015;6:1–10.
Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–4.
Davenport PW, Bolser DC, Vickroy T, Berry RB, Martin AD, Hey JA, et al. The effect of codeine on the Urge-to-Cough response to inhaled capsaicin. Pulm Pharmacol Ther. 2007;20(4):338–46.
Cohen J. A power primer. Quant Methods Psychol. 1992;112(1):155–9.
R Core Team. R: a language and environment for statistical computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2018. https://www.R-project.org/
Marik PE, Kaplan D. Aspiration pneumonia and dysphagia in the elderly. Chest. 2003;124(1):328–36.
Hely MA, Morris JGL, Traficante R, Reid WGJ, O’Sullivan DJ, Williamson PM. The Sydney multicentre study of Parkinson’s disease: progression and mortality at 10 years. J Neurol Neurosurg Psychiatry. 1999;67(3):300–7.
Fall P-A, Saleh A, Fredrickson M, Olsson J-E, Granérus A-K. Survival time, mortality, and cause of death in elderly patients with Parkinson’s disease. A 9-year follow-up: mortality in Parkinson’s disease. MovDisord. 2003;18(11):1312–6.
Yamada H, Masuda T, Okada M. Age-related EMG variables during maximum voluntary contraction. Percept Mot Skills. 2008;95:10–4.
Ketcham C, Stelmach G. Movement control in the older adult, vol. 3. Washington, D.C: National Academies Press; 2004. p. 305.
Elliott JE, Greising SM, Mantilla CB, Sieck GC. Functional impact of sarcopenia in respiratory muscles. Respir Physiol Neurobiol. 2016;226:137–46.
Kim J, Sapienza CM. Implications of expiratory muscle strength training for rehabilitation of the elderly: tutorial. JRRD. 2005;42(2):183–95.
Berardelli A. Pathophysiology of bradykinesia in Parkinson’s disease. Brain. 2001;124(11):2131–46.
Berardelli A, Sabra AF, Hallett M. Physiological mechanisms of rigidity in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1983;46(1):45–53.
van Geert P, van Dijk M. Focus on variability: new tools to study intra-individual variability in developmental data. Infant Behav Dev. 2002;25(4):340–74.
Funding
N/A.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Ethical Approval
All procedures performed were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all participants prior to enrollment in this research study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Borders, J.C., Brandimore, A.E. & Troche, M.S. Variability of Voluntary Cough Airflow in Healthy Adults and Parkinson’s Disease. Dysphagia 36, 700–706 (2021). https://doi.org/10.1007/s00455-020-10190-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-020-10190-3