Abstract
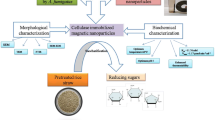
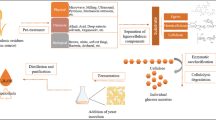
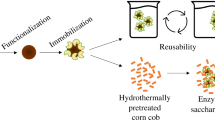
Enzymatic hydrolysis plays a pivotal role in transforming lignocellulosic biomass. Addressing alternate techniques to optimize the utilization of cellulolytic enzymes is one strategy to improve its efficiency and lower process costs. Cellulases are highly specific and environmentally benign biocatalysts that break down intricate polysaccharides into simple forms of sugars. In contrast to the most difficult and time-consuming enzyme immobilization processes, in this research, we studied simple, mild, and successful techniques for immobilization of pure cellulase on magnetic nanocomposites using glutaraldehyde as a linker and used in the application of sorghum residue biomass. Fe3O4 nanoparticles were coated with chitosan from the co-precipitation method, which served as an enzyme carrier. The nanoparticles were observed under XRD, Zeta Potential, FESEM, VSM, and FTIR. The size morphology results presented that the Cs@Fe3O4 have 42.2 nm, while bare nanoparticles (Fe3O4) have 31.2 nm in size. The pure cellulase reaches to 98.07% of loading efficiency and 71.67% of recovery activity at optimal conditions. Moreover, immobilized enzyme’s pH stability, thermostability, and temperature tolerance were investigated at suitable conditions. The kinetic parameters of free and immobilized enzyme were estimated as Vmax; 29 ± 1.51 and 27.03 ± 2.02 µmol min−1 mg−1, Km; 4.7 ± 0.49 mM and 2.569 ± 0.522 mM and Kcat; 0.13 s−1, and 0.89 s−1. Sorghum residue was subjected to 2% NaOH pre-treatment at 50 ℃. Pre-treated biomass contains cellulose of 64.8%, used as a raw material to evaluate the efficiency of reducing sugar during hydrolysis and saccharification of free and immobilized cellulase, which found maximum concentration of glucose 5.42 g/L and 5.12 g/L on 72 h. Thus, our study verifies the use of immobilized pure cellulase to successfully hydrolyze raw material, which is a significant advancement in lignocellulosic biorefineries and the reusability of enzymes.












Similar content being viewed by others
Data availability
Data will be made available on request.
References
Rose PK (2022) Bioconversion of agricultural residue into biofuel and high-value biochemicals: recent advancement. In: Zero waste biorefinery. Springer Nature Singapore, Singapore, pp. 233–268. https://doi.org/10.1007/978-981-16-8682-5_9
Stamenkoviić OS, Siliveru K, Veljković VB, Banković-Ilić IB, Tasić MB, Ciampitti IA, Dalović IG, Mitrović PM, Sikora VS, Prasad PVV (2020) Production of biofuels from sorghum. Renew Sustain Energy Rev 124:109769. https://doi.org/10.1016/j.rser.2020.109769
Zabed H, Sahu JN, Suely A, Boyce AN, Faruq G (2017) Bioethanol production from renewable sources: current perspectives and technological progress. Renew Sustain Energy Rev 71:475–501. https://doi.org/10.1016/j.rser.2016.12.076
Klasson KT, Boone SA (2021) Bioethanol fermentation of clarified sweet sorghum (Sorghum bicolor (L.) Moench) syrups sealed and stored under vegetable oil. Ind Crop Prod 163:113330. https://doi.org/10.1016/j.indcrop.2021.113330
Yucel C, Dweikat I, Yucel D, Gultekin R (2020) Bioethanol potential of different sweet sorghum genotypes grown under Mediterranean conditions. Fresenius Environ Bull 29(12):10356–10365
Sipos B, Réczey J, Somorai Z, Kádá Z, Dienes D, Réczey K (2009) Sweet sorghum as feedstock for ethanol production: enzymatic hydrolysis of steam-pretreated bagasse. Appl Biochem Biotechnol 153(1–3):151–162
Zhang C, Wen H, Chen C, Cai D, Fu C, Li P, Qin P, Tan T (2019) Simultaneous saccharification and juice co-fermentation for high-titer ethanol production using sweet sorghum stalk. Renew Energy 134:44–53. https://doi.org/10.1016/j.renene.2018.11.005
Sheikh ZUD, Bajar S, Devi A, Rose PK, Suhag M, Yadav A, Yadav DK, Deswal T, Kaur J, Kothari R, Pathania D (2023) Nanotechnology based technological development in biofuel production: current status and future prospects. Enzyme Microbial Technol 110304. https://doi.org/10.1016/j.enzmictec.2023.110304
Verma ML, Kumar P, Chandel H (2023) Nanobiotechnological routes in lignocellulosic waste pre-treatment for bio-renewables production. In: Gakkhar N, Kumar S, Sarma AK, Graham NT (eds) Recent advances in bio-energy research. ICRABR-2022 2022. Springer proceedings in energy. Springer, Singapore. https://doi.org/10.1007/978-981-99-5758-3_3
Rose PK, Dhull SB, Kidwai MK (2022) Cultivation of wild mushrooms using lignocellulosic biomass-based residue as a substrate. In: Wild mushrooms. CRC Press, pp. 493–520
Zhao X, Zhang L, Liu D (2012) Biomass recalcitrance. Part I: The chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuel Bioprod Bior 6(4):465–482
Rose PK, Poonia V, Kumar R, Kataria N, Sharma P, Lamba J, Bhattacharya P (2023) Congo red dye removal using modified banana leaves: adsorption equilibrium, kinetics, and reusability analysis. Groundw Sustain Dev 23:101005. https://doi.org/10.1016/j.gsd.2023.101005
Rose PK, Kumar R, Kumar R, Kumar M, Sharma P (2023) Congo red dye adsorption onto cationic amino-modified walnut shell: characterization, RSM optimization, isotherms, kinetics, and mechanism studies. Groundw Sustain Dev 21:100931. https://doi.org/10.1016/j.gsd.2023.100931
Rahikainen JL, Martin-Sampedro R, Heikkinen H, Rovio S, Marjamaa K, Tamminen T (2013) Inhibitory effect of lignin during cellulose bioconversion: the effect of lignin chemistry on non-productive enzyme adsorption. Bioresour Technol 133:270–278
Ávila PF, de Mélo AH, Goldbeck R (2023) Cello-oligosaccharides production from multi-stage enzymatic hydrolysis by lignocellulosic biomass and evaluation of prebiotic potential. Innov Food Sci Emerg Technol 85:103335
Xu C, Tong S, Sun L, Gu X (2023) Cellulase immobilization to enhance enzymatic hydrolysis of lignocellulosic biomass: an all-inclusive review. Carbohydr Polym 121319. https://doi.org/10.1016/j.carbpol.2023.121319
Revathi D, Ramalingam S (2023) A study on critical associations of media components on enhanced cellulase production from wild Trichoderma viride and cellulase immobilization on iron-oxide magnetic nanoparticles. J Environ Biol 44(1):27–33. https://doi.org/10.22438/jeb/441/MRN-5048
Sulman AM, Grebennikova OV, Karpenkov AY, Tikhonov BB, Molchanov VP, Matveeva VG et al (2023) Magnetic nanobiocatalysts based on immobilized cellulase. Chem Eng Trans 103:793–798. https://doi.org/10.3303/CET23103133
Verma ML, Barrow CJ (2015) Recent advances in feedstocks and enzyme-immobilised technology for effective transesterification of lipids into biodiesel. In: Kalia V (ed) Microbial factories. Springer, New Delhi. https://doi.org/10.1007/978-81-322-2598-0_6
Verma ML, Dhanya BS, Wang B, Thakur M, Rani V, Kushwaha R (2024) Bio-Nanoparticles mediated transesterification of algal biomass for biodiesel production. Sustainability 16:295. https://doi.org/10.3390/su16010295
Verma ML, Barrow CJ, Puri M et al (2013) Nanobiotechnology as a novel paradigm for enzyme immobilization and stabilization with potential applications in biodiesel production. Appl Microbiol Biotechnol 97:23–39
Galodiya MN, Chakma S (2024) Immobilization of enzymes on functionalized cellulose nanofibrils for bioremediation of antibiotics: degradation mechanism, kinetics, and thermodynamic study. Chemosphere 349:140803. https://doi.org/10.1016/j.chemosphere.2023.140803
Li C, Jiang S, Zhao X, Liang H (2017) Co-immobilization of enzymes and magnetic nanoparticles by metal-nucleotide hydrogel nanofibers for improving stability and recycling. Molecules 22:179. https://doi.org/10.3390/molecules22010179
Elamin NY, Modwi A, Abd El-Fattah W, Rajeh A (2023) Synthesis and structural of Fe3O4 magnetic nanoparticles and its effect on the structural optical, and magnetic properties of novel poly (methyl methacrylate)/polyaniline composite for electromagnetic and optical applications. Opt Mater 135:113323. https://doi.org/10.1016/j.optmat.2022.113323
Bilal M, Ashraf SS, Ferreira LFR, Cui J, Lou WY, Franco M (2020) Nanostructured materials as a host matrix to develop robust peroxidases-based nano biocatalytic systems. Int J Biol Macromol 162:1906–1923
Narisetty V, Tarafdar A, Bachan N, Madhavan A, Tiwari A, Chaturvedi P, Sindhu R (2023) An overview of cellulase immobilization strategies for biofuel production. BioEnergy Res 16(1):4–15
Nayeri MD, Nikkhah H, Zilouei H, Bazarganipour M (2023) Immobilization of cellulase on graphene oxide coated with NiFe2O4 and Fe3O4 for hydrolysis of rice straw. Cellulose 30(9):5549–5571. https://doi.org/10.1007/s10570-023-05207-7
Behshad Y, Pazhang M, Najavand S, Sabzi M (2023) Enhancing enzyme stability and functionality: covalent immobilization of trypsin on magnetic gum arabic modified Fe3O4 nanoparticles. Appl Biochem Biotechnol 1–18. https://doi.org/10.1007/s12010-023-04830-1
Ismail AM, Tiama TM, Farghaly A, Elhaes H, Ibrahim MA (2023) Assessment of the functionalization of chitosan/iron oxide nanoparticles. Biointerface Res Appl Chem 13(6):582
Kaur G, Taggar MS, Kalia A (2023) Cellulase-immobilized chitosan-coated magnetic nanoparticles for saccharification of lignocellulosic biomass. Environ Sci Pollut Res 1–21. https://doi.org/10.1007/s11356-023-27919-w
Verma ML, Dhanya BS, Rani V, Thakur M, Jeslin J, Kushwaha R (2020) Carbohydrate and protein based biopolymeric nanoparticles: current status and biotechnological applications. Int J Biol Macromol 154:390–412. https://doi.org/10.1016/j.ijbiomac.2020.03.105
Xu X, Chen T, Xu L, Lin J (2024) Immobilization of laccase on magnetic nanoparticles for enhanced polymerization of phenols. Enzyme Microb Technol 172:110331. https://doi.org/10.1016/j.enzmictec.2023.110331
Asar MF, Ahmad N, Husain Q (2020) Chitosan modified Fe3O4/graphene oxide nanocomposite as a support for high yield and stable immobilization of cellulase: its application in the saccharification of microcrystalline cellulose. Prep Biochem Biotechnol 50(5):460–467. https://doi.org/10.1080/10826068.2019.1706562
Verma ML, Sukriti Dhanya BS (2022) Synthesis and application of graphene-based sensors in biology: a review. Environ Chem Lett 20:2189–2212. https://doi.org/10.1007/s10311-022-01404-1
Ansari SA, Damanhory AA (2023) Biotechnological application of Aspergillus oryzae β-galactosidase immobilized on glutaraldehyde modified zinc oxide nanoparticles. Heliyon 9(2). https://doi.org/10.1016/j.heliyon.2023.e13089
Verma ML, Kumar S, Das A, Randhawa JS, Chamundeeswari M (2019) Enzyme immobilization on chitin and chitosan-based supports for biotechnological applications. In: Crini G, Lichtfouse E (eds) Sustainable agriculture reviews 35. Sustainable agriculture reviews, vol. 35. Springer, Cham. https://doi.org/10.1007/978-3-030-16538-3_4
Sharma B, Larroche C, Dussap CG (2020) Comprehensive assessment of 2G bioethanol production. Bioresour Technol 313:123630. https://doi.org/10.1016/j.biortech.2020.123630
Wirawan F, Cheng C-L, Lo Y-C, Chen C-Y, Chang J-S, Leu S-Y (2020) Continuous cellulosic bioethanol co-fermentation by immobilized Zymomonas mobilis and suspended Pichia stipitis in a two-stage process. Appl Energy 266:114871
Xie WL, Wang JL (2012) Immobilized lipase on magnetic chitosan microspheres for transesterification of soybean oil. Biomass Bioenerg 36:373–380
Zang L, Qiu J, Wu X, Zhang W, Sakai E, Wei Y (2014) Preparation of magnetic chitosan nanoparticles as support for cellulase immobilization. Ind Eng Chem Res 53:3448–3454
Ghose TK (1987) Measurement of cellulose activities. Pure Appl Chem 59(2):257–268
Selvarajan E, Mohanasrinivasan V (2015) Kinetic studies on exploring lactose hydrolysis potential of β galactosidase extracted from Lactobacillus plantarum HF571129. J Food Sci Technol 52:6206–6217
Punia P, Singh L (2024) Optimization of alkali pre-treatment of sweet sorghum [Sorghum bicolor (L.) Moench] residue to improve enzymatic hydrolysis for fermentable sugars. Waste Manage Bullet 2(1):131–141. https://doi.org/10.1016/j.wmb.2023.12.007
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker DLAP (2008) Determination of structural carbohydrates and lignin in biomass. Lab Anal Proc 1617(1):1–16
Trifoi AR, Matei E, Râpă M, Berbecaru AC, Panaitescu C, Banu I, Doukeh R (2023) Coprecipitation nanoarchitectonics for the synthesis of magnetite: a review of mechanism and characterization. React Kinet Mech Catal 136(6):2835–2874. https://doi.org/10.1007/s11144-023-02514-9
Sodipo BK, Noqta OA, Aziz AA, Katsikini M, Pinakidou F, Paloura EC (2023) Influence of capping agents on fraction of Fe atoms occupying octahedral site and magnetic property of magnetite (Fe3O4) nanoparticles by one-pot co-precipitation method. J Alloy Compd 938:168558. https://doi.org/10.1016/j.jallcom.2022.168558
Sanchez-Ramirez J, Martinez-Hernandez JL, Segura-Ceniceros P, Lopez G, Saade H, Medina-Morales MA, Ilyina A (2017) Cellulases immobilization on chitosan-coated magnetic nanoparticles: application for Agave Atrovirens lignocellulosic biomass hydrolysis. Bioprocess Biosyst Eng 40:9–22
Ding Yongling, Shirley Z. Shen, Huadong Sun, Kangning Sun, Futian Liu, Yushi Qi, Jun Yan (2015) Design and construction of polymerized-chitosan coated Fe3O4 magnetic nanoparticles and its application for hydrophobic drug delivery. Mater Sci Eng C 487–498. https://doi.org/10.1016/j.msec.2014.12.036
Karimova A, Shirinova H, Nargiz G, Nuriyeva S, Gahramanli L (2023) Preparation and characterization of magnetic iron oxide (Fe3O4) nanoparticles with different polymer coating agents
Kneller EF, Luborsky FE (1963) Particle size dependence of coercivity and remanence of single-domain particles. J Appl Phys 34:656–658. https://doi.org/10.1063/1.1729324
Kim DK, Zhang Y, Voit W, Rao KV, Muhammed M (2001) Synthesis and characterization of surfactant-coated superparamagnetic monodispersed iron oxide nanoparticles. J Magn Magn Mater 225:30–36. https://doi.org/10.1016/s0304-8853(00)01224-5
Podrepšek GH, Knez Z, Leitge M (2020) Development of chitosan functionalized magnetic nanoparticles with bioactive compounds. Nanomaterials 10:1913. https://doi.org/10.3390/nano10101913
Kuo CH, Liu YC, Chang CM, Chen JH, Chang C, Shieh CJ (2012) Optimum conditions for lipase immobilization on chitosan-coated Fe3O4 nanoparticles. Carbohydr Polym 87:2538–2545
Shanmugam S, Krishnaswamy S, Chandrababu R, Veerabagu U, Pugazhendhi A, Mathimani T (2020) Optimal immobilization of Trichoderma asperellum laccase on polymer coated Fe3O4@ SiO2 nanoparticles for enhanced biohydrogen production from delignified lignocellulosic biomass. Fuel 273:117777
John JA, Samuel MS, Selvarajan E (2023) Immobilized cellulase on Fe3O4/GO/CS nanocomposite as a magnetically recyclable catalyst for biofuel application. Fuel 333:126364
Ladole MR, Pokale PB, Varude VR, Belokar PG, Pandit AB (2021) One pot clarification and debittering of grapefruit juice using co-immobilized enzymes@ chitosan MNPs. Int J Biol Macromol 167:1297–1307
Chen Q, Liu D, Wu C, Yao K, Li Z, Shi N (2018) Co-immobilization of cellulase and lysozyme on amino-functionalized magnetic nanoparticles: an activity-tunable biocatalyst for extraction of lipids from microalgae. Bioresour Technol 263:317–324
Pandey AK, Negi S (2020) Enhanced cellulase recovery in SSF from Rhizopus oryzae SN5 and immobilization for multi-batch saccharification of carboxymethylcellulose. Biocatal Agric Biotechnol 26:101656
Paulraj Gundupalli M, Sahithi STA, Cheng Y-S, Tantayotai P, Sriariyanun M (2021) Differential effects of inorganic salts on cellulase kinetics in enzymatic saccharification of cellulose and lignocellulosic biomass. Bioprocess Biosyst Eng 44:2331–2344
Xia T-T, Liu C-Z, Hu J-H, Guo C (2016) Improved performance of immobilized laccase on amine-functioned magnetic Fe3O4 nanoparticles modified with polyethylenimine. Chem Eng J 295:201–206
Wen H, Chen H, Cai D, Gong P, Zhang T, Wu Z, Tan T (2018) Integrated in situ gas stripping–salting-out process for high-titer acetone–butanol–ethanol production from sweet sorghum bagasse. Biotechnol Biofuels 11:1–12. https://doi.org/10.1186/s13068-018-1137-5
Thanapimmetha A, Saisriyoot M, Khomlaem C, Chisti Y, Srinophakun PA (2019) Comparison of methods of ethanol production from sweet sorghum bagasse. Biochem Eng J 151:107352. https://doi.org/10.1016/j.bej.2019.107352
Su C, Qi L, Cai D, Chen B, Chen H, Zhang C, Si Z, Wang Z, Li G, Qin P (2020) Integrated ethanol fermentation and acetone-butanol-ethanol fermentation using sweet sorghum bagasse. Renew Energy 162:1125–1131. https://doi.org/10.1016/j.renene.2020.07.119
Loku UA, Ruplal C, Yanna L, John H, Watson DG (2015) Laboratory scale optimization of alkali pretreatment for improving enzymatic hydrolysis of sweet sorghum bagasse. Ind Crops Prod 74:977–986. https://doi.org/10.1016/j.indcrop.2015.05.044
Martins RP, Schmatz AA, de Freita LA, Mutton MJR, Brienzo M (2021) Solubilization of hemicellulose and fermentable sugars from bagasse, stalks, and leaves of sweet sorghum. Ind Crops Prod 170:113813
Acknowledgements
The authors are thankful to the Department of Environmental Science and Engineering, Guru Jambheshwar University of Science and Technology, Hisar, Haryana, India for providing multiple facilities for conducting this research.
Funding
There was no specific funding source for this conducted research work.
Author information
Authors and Affiliations
Contributions
PP and LS conceptualized the presented data. Pallavi performed all experiments, methodology and results under the supervision of Lakhvinder Singh. Pallavi wrote the manuscript with the contribution of Lakhvinder Singh. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Punia, P., Singh, L. Evaluation of free and immobilized cellulase on chitosan-modified magnetic nanoparticles for saccharification of sorghum residue. Bioprocess Biosyst Eng (2024). https://doi.org/10.1007/s00449-024-03010-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00449-024-03010-7