Abstract
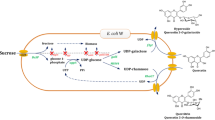
C-glycosylflavonoids have a number of pharmacological activities. An efficient method for the preparation of C-glycosylflavonoids is through metabolic engineering. Thus, it is important to prevent the degradation of C-glycosylflavonoids for producing C-glycosylflavonoids in the recombinant strain. In this study, two critical factors for the degradation of C-glycosylflavonoids were clarified. The quercetinase (YhhW) gene from Escherichia coli BL21(DE3) was expressed, purified, and characterized. YhhW effectively degraded quercetin 8-C-glucoside, orientin, and isoorientin, while the degradation of vitexin and isovitexin was not significant. Zn2+ can significantly reduce the degradation of C-glycosylflavonoids by inhibiting the activity of YhhW. pH was another key factor causing the degradation of C-glycosylflavonoids, and C-glycosylflavonoids were significantly degraded with pH exceeding 7.5 in vitro or in vivo. On this basis, two strategies, deleting YhhW gene from the genome of E. coli and regulating pH during the bioconversion, were developed to relieve the degradation of C-glycosylflavonoids. Finally, the total degradation rates for orientin and quercetin 8-C-glucoside decreased from 100 to 28% and 65% to 18%, respectively. The maximum yield of orientin reached 3353 mg/L with luteolin as substrate, and the maximum yield of quercetin 8-C-glucoside reached 2236 mg/L with quercetin as substrate. Therefore, the method described herein for relieving the degradation of C-glycosylflavonoids may be widely used for the biosynthesis of C-glycosylflavonoids in recombinant strains.
Graphical abstract









Similar content being viewed by others
Data availability
All relevant data are within the manuscript.
References
Chen L, Cao H, Huang Q, Xiao JB, Teng H (2022) Absorption, metabolism and bioavailability of flavonoids: a review. Crit Rev Food Sci Nutr 62:7730–7742
Burda S, Oleszek W (2001) Antioxidant and antiradical activities of flavonoids. J Agric Food Chem 49:2774–2779
Hwang SL, Shih PH, Yen GC (2012) Neuroprotective effects of citrus flavonoids. J Agric Food Chem 60:877–885
Kumar V, Jahan F, Mahajan RV, Saxena RK (2016) Efficient regioselective acylation of quercetin using Rhizopus oryzae lipase and its potential as antioxidant. Bioresour Technol 218:1246–1248
Li HB, Lyv YB, Zhou SH, Yu SQ, Zhou JW (2022) Microbial cell factories for the production of flavonoids-barriers and opportunities. Bioresour Technol 360:127538
Ren WY, Qiao ZH, Wang HW, Zhu L, Zhang L (2003) Flavonoids: promising anticancer agents. Med Res Rev 23:519–534
Yao LH, Jiang YM, Shi J, Tomas-Barberan FA, Datta N, Singanusong R, Chen SS (2004) Flavonoids in food and their health benefits. Plant Foods Hum Nutr 59:113–122
Rayyan S, Fossen T, Andersen OM (2005) Flavone C-glycosides from leaves of oxalis triangularis. J Agric Food Chem 53:10057–10060
Xiao JB, Capanoglu E, Jassbi AR, Miron A (2016) Advance on the flavonoid C-glycosides and health benefits. Crit Rev Food Sci Nutr 56:S29–S45
Rauter AP, Lopes RG, Martins A (2007) C-glycosylflavonoids: Identification, bioactivity and synthesis. Nat Prod Commun 2:1175–1196
Talhi O, Silva AMS (2012) Advances in C-glycosylflavonoid research. Curr Org Chem 16:859–896
Choi JS, Islam MN, Ali MY, Kim EJ, Kim YM, Jung HA (2014) Effects of C-glycosylation on anti-diabetic, anti-Alzheimer’s disease and anti-inflammatory potential of apigenin. Food Chem Toxicol 64:27–33
Courts FL, Williamson G (2015) The occurrence, fate and biological activities of C-glycosyl flavonoids in the human diet. Crit Rev Food Sci Nutr 55:1352–1367
Kim SM, Kang K, Jho EH, Jung YJ, Nho CW, Um BH, Pan CH (2011) Hepatoprotective effect of flavonoid glycosides from Lespedeza cuneata against oxidative stress induced by tert-butyl hyperoxide. Phytother Re 25:1011–1017
Peng X, Zheng Z, Cheng KW, Shan F, Ren GX, Chen F, Wang M (2008) Inhibitory effect of mung bean extract and its constituents vitexin and isovitexin on the formation of advanced glycation endproducts. Food Chem 106:475–481
Hu C, Zhang Y, Kitts DD (2000) Evaluation of antioxidant and prooxidant activities of bamboo Phyllostachys nigra var. Henonis leaf extract in vitro. J Agric Food Chem 48:3170–3176
Byrne PF, Darrah LL, Snook ME, Wiseman BR, Barry BD (1996) Maize silk-browning, maysin content, and antibiosis to the corn earworm, Helicoverpa zea (Boddie). Maydica 41:13–18
Cheynier V, Comte G, Davies KM, Lattanzio V, Martens S (2013) Plant phenolics: recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol Biochem 72:1–20
Zhang Y, Jiao JJ, Liu CM, Wu XQ, Zhang Y (2008) Isolation and purification of four flavone C-glycosides from antioxidant of bamboo leaves by macroporous resin column chromatography and preparative high-performance liquid chromatography. Food Chem 107(3):1326–1336
Oyama K, Yoshida K, Kondo T (2011) Recent progress in the synthesis of flavonoids: from monomers to supra-complex molecules. Curr Org Chem 15:2567–2607
Wang Y, Chen S, Yu O (2011) Metabolic engineering of flavonoids in plants and microorganisms. Appl Microbiol Biotechnol 91:949–956
Mrudulakumari Vasudevan U, Lee EY (2020) Flavonoids, terpenoids, and polyketide antibiotics: role of glycosylation and biocatalytic tactics in engineering glycosylation. Biotechnol Adv 41:107550
Xie K, Zhang X, Sui S, Ye F, Dai J (2020) Exploring and applying the substrate promiscuity of a C-glycosyltransferase in the chemo-enzymatic synthesis of bioactive C-glycosides. Nat Commu 11:5162
Cao H, Chen XQ, Jassbi AR, Xiao JB (2015) Microbial biotransformation of bioactive flavonoids. Biotechnol Adv 33:214–223
Shrestha A, Pandey RP, Dhakal D, Parajuli P, Sohng JK (2018) Biosynthesis of flavone C-glucosides in engineered Escherichia coli. Appl Microbiol Biotechnol 102:1251–1267
Pei JJ, Sun Q, Zhao LG, Shi H, Tang F, Cao F (2019) Efficient Biotransformation of luteolin to isoorientin through adjusting induction strategy, controlling acetic acid, and increasing UDP-glucose supply in Escherichia coli. J Agric Food Chem 67:331–340
He JB, Zhao P, Hu ZM, Liu S, Kuang Y, Zhang M, Li B, Yun CH, Qiao X, Ye M (2019) Molecular and structural characterization of a promiscuous C-glycosyltransferase from Trollius chinensis. Angew Chem Int Ed Engl 58:11513–11520
Wu YB, Wang H, Liu Y, Zhao LG, Pei JJ (2022) An efficient preparation and biocatalytic synthesis of novel C-glycosylflavonols kaempferol 8-C-glucoside and quercetin 8-C-glucoside through using resting cells and macroporous resins. Biotechnol Biofuels Bioprod 15(1):1–6
Braune A, Blaut M (2012) Intestinal bacterium Eubacterium cellulosolvens deglycosylates flavonoid C- and O-glucosides. Appl Environ Microbiol 78:8151–8153
Kim M, Lee J, Han J (2015) Deglycosylation of isoflavone C-glycosides by newly isolated human intestinal bacteria. J Sci Food Agric 95:1925–1931
Mori T, Kumano T, He HB, Watanabe S, Senda M, Moriya T, Adachi N, Hori S, Terashita Y, Kawasaki M (2021) C-Glycoside metabolism in the gut and in nature: Identification, characterization, structural analyses and distribution of C-C bond-cleaving enzymes. Nat Commun 12(1):6294
Braune A, Gutschow M, Blaut M (2019) An NADH-dependent reductase from eubacterium ramulus catalyzes the stereospecific heteroring cleavage of flavanones and flavanonols. Appl Environ Microbiol 85(19):e01233
Braune A, Engst W, Blaut M (2016) Identification and functional expression of genes encoding flavonoid O- and C-glycosidases in intestinal bacteria. Environ Microbiol 18:2117–2129
Pillai BVS, Swarup S (2002) Elucidation of the flavonoid catabolism pathway in Pseudomonas putida PML2 by comparative metabolic profiling. Appl Environ Microbiol 68:143–151
Rao JR, Cooper JE (1994) Rhizobia catabolize nod gene-inducing flavonoids via C-ring fission mechanisms. J Bacteriol 176:5409–5413
Marin AM, Souza EM, Pedrosa FO, Souza LM, Sassaki GL, Baura VA, Yates MG, Wassem R, Monteiro RA (2013) Naringenin degradation by the endophytic diazotroph Herbaspirillum seropedicae SmR1. Microbiology-Sgm 159:167–175
Adams M, Jia ZC (2005) Structural and biochemical analysis reveal pirins to possess quercetinase activity. J Biol Chem 280:28675–28682
Zangoui P, Vashishtha K, Mahadevan S (2015) Evolution of aromatic beta-glucoside utilization by successive mutational steps in Escherichia coli. J Bacteriol 197(4):710–716
Jiang Y, Chen B, Duan CL, Sun BB, Yang JJ, Yang S (2015) Multigene editing in the Escherichia coli genome via the CRISPR-Cas9 system. Appl Environ Microbiol 81:2506–2514
Li Q, Sun BB, Chen J, Zhang YW, Jiang Y, Yang S (2021) A modified pCas/pTargetF system for CRISPR-Cas9-assisted genome editing in Escherichia coli. Acta Biochim Biophys Sin 53(5):620–627
Pacifico S, Scognamiglio M, D’Abrosca B, Piccolella S, Tsafantakis N, Gallicchio M, Ricci A, Fiorentino A (2010) Spectroscopic characterization and antiproliferative activity on HepG2 human hepatoblastoma cells of flavonoid C-glycosides from Petrorhagia velutina. J Nat Prod 73:1973–1978
Yan F, Yang Y, Yu L, Zheng X (2017) Effects of C-glycosides from apios americana leaves against oxidative stress during hyperglycemia through regulating mitogen-activated protein kinases and nuclear factor erythroid 2-related factor 2. J Agric Food Chem 65:7457–7466
Guo B, Zhang Y, Hicks G, Huang X, Li R, Roy N, Jia Z (2019) Structure-dependent modulation of substrate binding and biodegradation activity of pirin proteins toward plant flavonols. ACS Chem Biol 14:2629–2640
Antonczak S, Fiorucci S, Golebiowski J, Cabrol-Bass D (2009) Theoretical investigations of the role played by quercetinase enzymes upon the flavonoids oxygenolysis mechanism. Phys Chem Chem Phys 11(10):1491–1501
Acknowledgements
This work was supported by the National Key R&D Program of China (2022YFD2200905) and the National Natural Science Foundation of China (21978135).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, S., Liu, J., Zhao, L. et al. Engineering Escherichia coli for efficient and economic production of C-glycosylflavonoids by deleting YhhW and regulating pH. Bioprocess Biosyst Eng 46, 1251–1264 (2023). https://doi.org/10.1007/s00449-023-02893-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-023-02893-2