Abstract
Oxidoreductase is one of the most important biocatalysts for the synthesis of various chiral compounds. However, their whole-cell activity is frequently affected by an insufficient supply of expensive nicotinamide cofactors. This study aimed to overcome such shortcomings by developing a combination fermentation strategy for simultaneously increasing intracellular NADP(H) level, biomass, and glufosinate dehydrogenase activity in E. coli. The results showed that the feeding mode of NAD(H) synthesis precursor and lactose inducer had essential effects on the accumulation level of intracellular NADPH. Adding 40 mg L−1 of L-aspartic acid to the medium increased the intracellular NADP(H) concentration by 36.3%. Under the pH-stat feeding mode and adding 0.4 g L−1 h−1 lactose, the NADP(H) concentration, biomass, and GluDH activity in the 5-L fermenter reached 445.7 μmol L−1, 21.7 gDCW L−1, and 8569.3 U L−1, respectively. As far as we know, this is the highest reported activity of GluDH in the fermentation broth. Finally, the 5000-L fermenter was successfully scaled up to use this fermentation approach. The combination fermentation strategy might serve as a useful approach for the high-activity fermentation of other NADPH-dependent oxidoreductases.




Similar content being viewed by others
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Vidal LS, Kelly CL, Mordaka PM, Heap JT (2018) Review of NAD(P)H-dependent oxidoreductases: properties, engineering and application. Biochimica Et Biophysica Acta-Prot Proteom 1866:327–347. https://doi.org/10.1016/j.bbapap.2017.11.005
Fernandez-Lucas J (2021) New trends in industrial biocatalysis. Biotech Adv. 51:107782. https://doi.org/10.1016/j.biotechadv.2021.107782
Li S, Ye Z, Moreb EA, Hennigan JN, Castellanos DB, Yang T, Lynch MD (2021) Dynamic control over feedback regulatory mechanisms improves NADPH flux and xylitol biosynthesis in engineered E. coli. Metab Eng 64:26–40. https://doi.org/10.1016/j.ymben.2021.01.005
Tesfay MA, Win X, Lin HB, Liu YJ, Li C, Lin JQ, Lin JQ (2021) Efficient L-xylulose production using whole-cell biocatalyst with NAD(+) regeneration system through co-expression of xylitol dehydrogenase and NADH oxidase in Escherichia coli. Biochem Eng J 175:108137. https://doi.org/10.1016/j.bej.2021.108137
Xue YP, Cao CH, Zheng YG (2018) Enzymatic asymmetric synthesis of chiral amino acids. Chem Soc Rev 47:1516–1561. https://doi.org/10.1039/c7cs00253j
Cheng F, Wei L, Wang CJ, Xue YP, Zheng YG (2022) Formate dehydrogenase and its application in biomanufacturing of chiral chemicals. Chin J Biotechnol 38:632–649. https://doi.org/10.13345/j.cjb.210278
Morrison CS, Armiger WB, Dodds DR, Dordicka JS, Koff MAG (2018) Improved strategies for electrochemical 1,4-NAD(P)H2 regeneration: A new era of bioreactors for industrial biocatalysis. Biotechnol Adv 36:120–131. https://doi.org/10.1016/j.biotechadv.2017.10.003
Liu J, Li H, Zhao G, Caiyin Q, Qiao J (2018) Redox cofactor engineering in industrial microorganisms: strategies, recent applications and future directions. J Ind Microbiol Biotechnol 45:313–327. https://doi.org/10.1007/s10295-018-2031-7
Yang LY, Mu XQ, Nie Y, Xu Y (2021) Improving the production of NAD(+) via multi-strategy metabolic engineering in Escherichia coli. Metab Eng 64:122–133. https://doi.org/10.1016/j.ymben.2021.01.012
Yuan L, Qin YL, Zou ZC, Appiah B, Huang H, Yang ZH, Qun C (2022) Enhancing intracellular NADPH bioavailability through improving pentose phosphate pathway flux and its application in biocatalysis asymmetric reduction reaction. J Biosci Bioeng 134:528–533. https://doi.org/10.1016/j.jbiosc.2022.08.010
Berrios- Rivera SJ, San KY, Bennett GN (2002) The effect of NAPRTase overexpression on the total levels of NAD, the NADH/NAD(+) ratio, and the distribution of metabolites in Escherichia coli. Metab Eng 4:238–247. https://doi.org/10.1006/mben.2002.0229
Sun W, Shahrajabian MH, Lin M (2022) Research progress of fermented functional foods and protein factory-microbial fermentation technology. Fermentation-Basel 8:688. https://doi.org/10.3390/fermentation8120688
Wang C, Xin FX, Kong XP, Zhao J, Dong WL, Zhang WM, Ma JF, Wu H, Jiang M (2018) Enhanced isopropanol-butanol-ethanol mixture production through manipulation of intracellular NAD(P)H level in the recombinant Clostridium acetobutylicum XY16. Biotechnol Biofuels 11:12. https://doi.org/10.1186/s13068-018-1024-0
Ping Y, Liu YZ, Jian M, Qili Z, Qing CW (2022) Efficient regeneration of NADPH based on gluco kinase and glucose-6-phosphate dehydrogenase. J Chin Instit Food Sci Technol 22:163–172. https://doi.org/10.16429/j.1009-7848.2022.08.018
Han Q, Eiteman MA (2018) Enhancement of NAD(H) pool for formation of oxidized biochemicals in Escherichia coli. J Ind Microbiol Biotechnol 45:939–950. https://doi.org/10.1007/s10295-018-2072-y
Oeggl R, Neumann T, Gatgens J, Romano D, Noack S, Rother D (2018) Citrate as cost-efficient NADPH regenerating agent. Front Bioeng Biotechnol 6:196. https://doi.org/10.3389/fbioe.2018.00196
San KY, Bennett GN, Berrios-Rivera SJ, Vadali RV, Yang YT, Horton E, Rudolph FB, Sariyar B, Blackwood K (2002) Metabolic engineering through cofactor manipulation and its effects on metabolic flux redistribution in Escherichia coli. Metab Eng 4:182–192. https://doi.org/10.1006/mben.2001.0220
Zou C, Duan XG, Wu J (2014) Enhanced extracellular production of recombinant Bacillus deramificans pullulanase in Escherichia coli through induction mode optimization and a glycine feeding strategy. Biores Technol 172:174–179. https://doi.org/10.1016/j.biortech.2014.09.035
Zou SP, Jiang ZT, Tang H, Cheng F, Xue YP, Zheng YG (2022) Preparation of cross-linked cell aggregates (CLCAs) of recombinant E coli harboring glutamate dehydrogenase and glucose dehydrogenase for efficient asymmetric synthesis of L-phosphinothricin. Biochem Eng J 184:108468. https://doi.org/10.1016/j.bej.2022.108468
Cheng F, Li H, Zhang K, Li QH, Xie D, Xue YP, Zheng YG (2020) Tuning amino acid dehydrogenases with featured sequences for L-phosphinothricin synthesis by reductive amination. J Biotechnol 312:35–43. https://doi.org/10.1016/j.jbiotec.2020.03.001
Cheng F, Li QH, Zhang HY, Wei L, Zhang JM, Li JM, Xue YP, Zheng YG (2021) Simultaneous directed evolution of coupled enzymes for efficient asymmetric synthesis of L-phosphinothricin. Appl Environ Microbiol 87:e02563-e2620. https://doi.org/10.1128/aem.02563-20
Fang S, Li J, Liu L, Du G, Chen J (2011) Overproduction of alkaline polygalacturonate lyase in recombinant Escherichia coli by a two-stage glycerol feeding approach. Bioresour Technol 102:10671–10678. https://doi.org/10.1016/j.biortech.2011.09.020
Chua LH, Tan SC, Liew MWO (2018) Process intensification of core streptavidin production through high-cell-density cultivation of recombinant E. coli and a temperature-based refolding method. J Biotechnol 276–277:34–41. https://doi.org/10.1016/j.jbiotec.2018.04.012
Hori C, Yamazaki T, Ribordy G, Takisawa K, Matsumoto K, Ooi T, Zinn M, Taguchi S (2019) High-cell density culture of poly(lactate-co-3-hydroxybutyrate)-producing Escherichia coli by using glucose/xylose-switching fed-batch jar fermentation. J Biosci Bioeng 127:721–725. https://doi.org/10.1016/j.jbiosc.2018.11.006
Xu JM, Zhang K, Cao HT, Li H, Cheng F, Cao CH, Xue YP, Zheng YG (2020) Development of a biocatalytic cascade for synthesis of 2-oxo-4-(hydroxymethylphosphinyl) butyric acid in one pot. Biocatal Biotransform 39:190–197. https://doi.org/10.1080/10242422.2020.1797697
Duan X, Zhang X, Shen Z, Su E, Zhao L, Pei J (2019) Efficient production of aggregation prone 4-alpha-glucanotransferase by combined use of molecular chaperones and chemical chaperones in Escherichia coli. J Biotechnol 292:68–75. https://doi.org/10.1016/j.jbiotec.2019.01.014
Riesenberg D, Schulz V, Knorre WA, Pohl HD, Korz D, Sanders EA, Ross A, Deckwer WD (1991) High cell density cultivation of Escherichia coli at controlled specific growth rate. J Biotechnol 20:17–28. https://doi.org/10.1016/0168-1656(91)90032-q
Vera A, Montalban NG, Aris A, Villaverde A (2007) The conformational quality of insoluble recombinant proteins is enhanced at low growth temperatures. Biotechnol Bioeng 96:1101–1106. https://doi.org/10.1002/bit.21218
Kaur J, Kumar A, Kaur J (2018) Strategies for optimization of heterologous protein expression in E-coli: Roadblocks and reinforcements. Int J Biol Macromol 106:803–822. https://doi.org/10.1016/j.ijbiomac.2017.08.080
Dragosits M, Frascotti G, Granger LB, Vazquez F, Giuliani M, Baumann K, Carmona ER, Tokkanen J, Parrilli E, Wiebe MG, Kunert R, Maurer M, Gasser B, Sauer M, Branduardi P, Pakula T, Saloheimo M, Penttila M, Ferrer P, Tutino ML, Villaverde A, Porro D, Mattanovich D (2011) Influence of growth temperature on the production of antibody Fab fragments in different microbes: A host comparative analysis. Biotechnol Prog 27:38–46. https://doi.org/10.1002/btpr.524
Zou SP, Wang ZJ, Zhao K, Zhang B, Liu ZQ, Niu K, Zheng YG (2021) High-level production of d-pantothenic acid from glucose by fed-batch cultivation of Escherichia coli. Biotechnol Appl Biochem 68:1227–1235. https://doi.org/10.1002/bab.2044
Joachim M, Schäfer JG, Gerlach D, Czermak P (2018) High cell density cultivation of Δgor/ΔtrxB E. coli in a chemically defined minimal medium with an enhanced iron concentration. Process Biochem 73:1–5. https://doi.org/10.1016/j.procbio.2018.07.022
Chi L, Wei JJ, Hou JC, Wang JY, Hu XL, He PX, Wei T (2020) Optimizing the DO-stat protocol for enhanced production of thermostable pullulanase in Escherichia coli by using oxygen control strategies. J Food Biochem 44:13173. https://doi.org/10.1111/jfbc.13173
Zou SP, Zhao K, Tang H, Zhang Z, Zhang B, Liu ZQ, Zheng YG (2021) Improved production of D-pantothenic acid in Escherichia coli by integrated strain engineering and fermentation strategies. J Biotechnol 339:65–72. https://doi.org/10.1016/j.jbiotec.2021.07.014
Chen SY, Wei YH, Chang JS (2007) Repeated pH-stat fed-batch fermentation for rhamnolipid production with indigenous Pseudomonas aeruginosa S2. Appl Microbiol Biotechnol 76:67–74. https://doi.org/10.1007/s00253-007-0980-2
Jin LQ, Peng F, Liu HL, Cheng F, Jia DX, Xu JM, Zq L, Xue YP, Zheng YG (2019) Asymmetric biosynthesis of L-phosphinothricin by a novel transaminase from Pseudomonas fluorescens ZJB09-108. Process Biochem 85:60–67. https://doi.org/10.1016/j.procbio.2019.07.010
Cheng F, Zhang JM, Jiang ZT, Wu XH, Xue YP, Zheng YG (2022) Development of an NAD(H)-driven biocatalytic system for asymmetric synthesis of chiral amino acids. Adv Synth Catal 364:1450–1459. https://doi.org/10.1002/adsc.202101441
Zou SP, Zhong W, Xia CJ, Gu YN, Niu K, Zheng YG, Shen YC (2015) Mutagenesis breeding of high echinocandin B producing strain and further titer improvement with culture medium optimization. Bioprocess Biosyst Eng 38:1845–1854. https://doi.org/10.1007/s00449-015-1425-4
Post MJ, Levenberg S, Kaplan DL, Genovese N, Fu JA, Bryant CJ, Negowetti N, Verzijden K, Moutsatsou P (2020) Scientific, sustainability and regulatory challenges of cultured meat. Nature Food 1:403–415. https://doi.org/10.1038/s43016-020-0112-z
Singh V, Haque S, Niwas R, Srivastava A, Pasupuleti M, Tripathi CKM (2017) Strategies for fermentation medium optimization: An in-depth review. Front Microbiol 7:02087. https://doi.org/10.3389/fmicb.2016.02087
Wu Y, Li H, Zhang XM, Gong JS, Li H, Rao ZM, Shi JS, Xu ZH (2015) Improvement of NADPH-dependent P450-mediated biotransformation of 7alpha,15alpha-diOH-DHEA from DHEA by a dual cosubstrate-coupled system. Steroids 101:15–20. https://doi.org/10.1016/j.steroids.2015.05.005
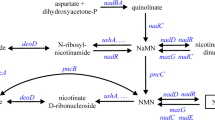
Liao Z, Yang X, Fu H, Wang J (2019) The significance of aspartate on NAD(H) biosynthesis and ABE fermentation in Clostridium acetobutylicum ATCC 824. AMB Express 9:142. https://doi.org/10.1186/s13568-019-0874-6
Knepper A, Schleicher M, Klauke M, Botz DW (2008) Enhancement of the NAD(P)(H) Pool in Saccharomyces cerevisiae. Eng Life Sci 8:381–389. https://doi.org/10.1002/elsc.200800031
Moon SJ, Dong WT, Stephanopoulos GN, Sikes HD (2020) Oxidative pentose phosphate pathway and glucose anaplerosis support maintenance of mitochondrial NADPH pool under mitochondrial oxidative stress. Bioeng Translat Med 5:10184. https://doi.org/10.1002/btm2.10184
Heuser F, Schroer K, Lutz S, Meyer SB, Sahm H (2007) Enhancement of the NAD(P)(H) pool in Escherichia coli for biotransformation. Eng Life Sci 7:343–353. https://doi.org/10.1002/elsc.200720203
Magnani F, Mattevi A (2019) Structure and mechanisms of ROS generation by NADPH oxidases. Curr Opin Struct Biol 59:91–97. https://doi.org/10.1016/j.sbi.2019.03.001
Meng JZ, Chen FZ, Wang QY, Huang RB (2006) Recombinant alginate synthase engineering bacteria pH-stat high density fermentation process study. Sci Technol Food Indust. 27:125–128. https://doi.org/10.13386/j.issn1002-0306.2006.08.034
Kim JH, Kim SW, Nguyen DQA, Li H, Kim SB, Seo YG, Yang JK, Chung IY, Kim DH, Kim CJ (2009) Production of beta-carotene by recombinant Escherichia coli with engineered whole mevalonate pathway in batch and fed-batch cultures. Biotechnol Bioprocess Eng 14:559–564. https://doi.org/10.1007/s12257-008-0230-1
Blommel PG, Becker KJ, Duvnjak P, Fox BG (2007) Enhanced bacterial protein expression during auto-induction obtained by alteration of lac repressor dosage and medium composition. Biotechnol Prog 23:585–598. https://doi.org/10.1021/bp070011x
Kilikian BV, Suarez ID, Liria CW, Gombert AK (2000) Process strategies to improve heterologous protein production in Escherichia coli under lactose or IPTG induction. Process Biochem 35:1019–1025. https://doi.org/10.1016/s0032-9592(00)00137-0
Dvorak P, Chrast L, Nikel PI, Fedr R, Soucek K, Sedlackova M, Chaloupkova R, deLorenzo V, Prokop Z, Damborsky J (2015) Exacerbation of substrate toxicity by IPTG in Escherichia coli BL21 (DE3) carrying a synthetic metabolic pathway. Microb Cell Fact 14:15. https://doi.org/10.1186/s12934-015-0393-3
Pei XL, Wang QY, Li CL, Qiu XF, Xie KL, Huang LF, Wang AM, Zeng ZW, Xie T (2011) Efficient production of a thermophilic 2-Deoxyribose-5-Phosphate aldolase in glucose-limited fed-batch cultivations of Escherichia coli by continuous lactose induction strategy. Appl Biochem Biotechnol 165:416–425. https://doi.org/10.1007/s12010-011-9261-8
Lee C, Sun WJ, Burgess BW, Junker BH, Reddy J, Buckland BC, Greasham RL (1997) Process optimization for large-scale production of TGF-alpha-PE40 in recombinant Escherichia coli: Effect of medium composition and induction timing on protein expression. J Ind Microbiol Biotechnol 18:260–266. https://doi.org/10.1038/sj.jim.2900382
Acknowledgements
This research/project is supported by the National Natural Science Foundation of China (NO. 21978268) and the National Key Research and Development Program of China (grant 2019YFA0905000).
Author information
Authors and Affiliations
Contributions
SZ: conceptualization, investigation, formal analysis, data curation, writing—original draft. JL: investigation, formal analysis, validation, writing—original draft. BZ: investigation, data curation. XL: investigation, data curation. ZJ: investigation, data curation. YX: writing—review and editing, funding acquisition. YZ: writing—review and editing, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
All authors have declared no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zou, S., Lu, J., Zhang, B. et al. A combination fermentation strategy for simultaneously increasing cellular NADP(H) level, biomass, and enzymatic activity of glufosinate dehydrogenase in Escherichia coli. Bioprocess Biosyst Eng 46, 867–878 (2023). https://doi.org/10.1007/s00449-023-02871-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-023-02871-8