Abstract
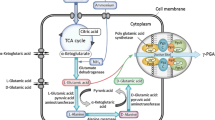
As an important five-carbon platform chemical to synthesize polyesters and polyamides, glutaric acid is widely used in numerous biochemical fields such as consumer goods, textile, and footwear industries. However, the application of glutaric acid is limited by the low yield of its bio-production. In this study, a metabolically engineered Escherichia coli LQ-1 based on 5-aminovalerate (AMV) pathway was used for glutaric acid fed-batch fermentation. Given the significance of nitrogen source in the bio-production of glutaric acid by AMV pathway, a novel nitrogen source feeding strategy feedbacked by real-time physiological parameters was proposed after evaluating the effects of nitrogen source feeding (such as ammonia and ammonium sulfate) on glutaric acid bio-production. Under the proposed nitrogen source feeding strategy, a significantly improved glutaric acid production of 53.7 g L–1 was achieved in a 30 L fed-batch fermentation by the metabolically engineered E. coli LQ-1, which was an improvement of 52.1% over pre-optimization. Additionally, a higher conversion rate of 0.64 mol mol–1 (glutaric acid/glucose) was obtained compared with the previously reported bio-production of glutaric acid with E. coli. These results indicated that the nitrogen source feeding strategy proposed in this study will be useful for achieving the efficient and sustainable bio-based production of glutaric acid.








Similar content being viewed by others
Data availability
All relevant data are within the manuscript.
References
Navarro E, Puiggali J, Subirana JA (1997) The structure of nylon 12, 5 is characterized by two hydrogen bond directions as are other polyamides derived from glutaric acid. Polymer 38:3429–3432
Navarro E, Franco L, Subirana JA, Puiggali J (1995) Nylon 65 has a unique structure with two directions of hydrogen bonds. Macromolecules 28:8742–8750
Ricart A, Puiggalí J, Franco L, Morales-Gámez L (2012) Study on the brill transition and melt crystallization of nylon 65: a polymer able to adopt a structure with two hydrogen-bonding directions. Eur Polym J 46:2063–2077
Rohles CM, Gläser L, Kohlstedt M, Gießelmann G, Pearson S, Campo AL, Becker J, Wittmann C (2018) A bio-based route to the carbon-5 chemical glutaric acid and to bionylon-6,5 using metabolically engineered Corynebacterium glutamicum. Green Chem 20:4462–4474
Mishra MK, Varughese S, Ramamurty U, Desiraju GR (2013) Odd-even effect in the elastic modulii of alpha, omega-alkanedicarboxylic acids. J Am Chem Soc 135:8121–8124
Li JQ, Gao XQ, Ding SM, Qian XR, Ma YG (2011) Separation and preparation of glutaric acid from mixed dicarboxylic acid. Chem Ind Eng 28:53–56
Chen H, Dai WL, Gao RH, Cao Y, Fan KN (2007) New green catalytic manufacture of glutaric acid from the oxidation of cyclopentane-1,2-diol with aqueous hydrogen peroxide. Appl Catal A 328:226–236
Vafaeezadeh M, Hashemi MM (2016) A non-cyanide route for glutaric acid synthesis from oxidation of cyclopentene in the ionic liquid media. Process Saf Environ 100:203–207
Yu JL, Xia XX, Zhong JJ, Qian ZG (2017) A novel synthetic pathway for gultarate production in recombinant Escherichia coli. Process Biochem 59:167–171
Yu JL, Xia XX, Zhong JJ, Qian ZG (2017) Enhanced production of C5 dicarboxylic acids by aerobic-anaerobic shift in fermentation production of engineered Escherichia coli. Process Biochem 62:53–58
Wang J, Wu Y, Sun X, Yuan QP, Yan YJ (2017) De novo biosynthesis of glutarate via a-keto acid carbon chain extension and decarboxylation pathway in Escherichia coli. ACS Synth Biol 6:1922–1930
Zhao M, Li G, Deng Y (2018) Engineering Escherichia coli for glutarate production as the C5 platform backbone. Appl Environ Microb 84:814–818
Revelles O, Espinosa-Urgel M, Fuhrer T, Sauer U, Ramos JL (2005) Multiple and interconnected pathways for L-lysine catabolism in Pseudomonas putida KT2440. J Bacteriol 187:7500–7510
Rohles CM, Gießelmann G, Kohlstedt M, Wittmann C, Becker J (2016) Systems metabolic engineering of Corynebacterium glutamicum for the production of the carbon-5 platform chemicals 5-aminovalerate and glutarate. Microb Cell Fact 15:1–13
Kim HT, Khang TU, Baritugo KA, Hyun SM, Kang KH, Jung SH, Song BK, Park K, Oh MK, Kim GB, Kim HU, Lee SY, Park SJ, Joo JC (2019) Metabolic engineering of Corynebacterium glutamicum for the production of glutaric acid, a C5 dicarboxylic acid platform chemical. Metab Eng 51:99–109
Han T, Kim GB, Lee SY (2020) Glutaric acid production by systems metabolic engineering of an L-lysine–overproducing Corynebacterium glutamicum. Proc Natl Acad Sci U S A 117:30328–30334
Adkins J, Jordan J, Nielsen DR (2013) Engineering Escherichia coli for renewable production of the 5-carbon polyamide building-blocks 5- aminovalerate and glutarate. Biotechnol Bioeng 110:1726–1734
Li WN, Ma L, Shen XL, Wang J, Feng Q, Liu LX, Zheng GJ, Yan YJ, Sun XX, Yuan QP (2019) Targeting metabolic driving and intermediate influx in L-lysine catabolism for high-level glutarate production. Nat Commun 10:3337
Wang Y, Chu J, Zhuang YP, Wang YH, Xia JY, Zhang SL (2009) Industrial bioprocess control and optimization in the context of systems biotechnology. Biotechnol Adv 27:989–995
Wang ZJ, Wang HY, Li YL, Chu J, Huang MZ, Zhuang YP, Zhang SL (2010) Improved vitamin B12 production by step-wise reduction of oxygen uptake rate under dissolved oxygen limiting level during fermentation process. Bioresour Technol 101:2845–2852
Sáez-Plaza P, Michałowski T, Navas MJ, Asuero AG, Wybraniec S (2013) An overview of the Kjeldahl method of nitrogen determination. Part I. early history, chemistry of the procedure, and titrimetric finish. Crit Rev Anal Chem 43:178–223
Magasanik B, Kaiser CA (2002) Nitrogen regulation in Saccharomyces cerevisiae. Gene 290:1–18
Wong KH, Hynes MJ, Davis MA (2008) Recent advances in nitrogen regulation: a comparison between Saccharomyces cerevisiae and filamentous fungi. Eukaryot Cell 7:917–925
Chen Y, Wang ZJ, Chu J, Xi BL, Zhuang YP (2015) The glucose RQ-feedback control leading to improved erythromycin production by a recombinant strain Saccharopolyspora erythraea ZL1004 and its scale-up to 372 m3 fermenter. Bioprocess Biosyst Eng 38:105–112
Lu F, Wang ZJ, Zhao W, Chu J, Zhuang YP (2016) A simple novel approach for real-time monitoring of sodium gluconate production by on-line physiological parameters in batch fermentation by Aspergillus niger. Bioresour Technol 202:133–141
Acknowledgements
This work was supported by grants from National Key Research and Development Program of China (2022YFC2106204).
Author information
Authors and Affiliations
Contributions
WB: Investigation, Data curation, Validation, Writing-original draft. CC: Investigation, Visualization, Data curation. TW: Formal analysis, Validation, Writing-review and editing. PY: Validation, Writing-review and editing. NL: Conceptualization, Resources, Writing-review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bi, W., Chen, C., Wang, T. et al. Efficient bio-production of glutaric acid by a metabolically engineered Escherichia coli LQ-1 based on a novel nitrogen source feeding strategy. Bioprocess Biosyst Eng 46, 717–725 (2023). https://doi.org/10.1007/s00449-023-02856-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-023-02856-7