Abstract
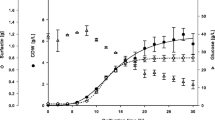
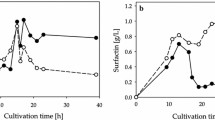
Surfactin biosurfactant produced by Bacillus sp. has been studied, because it has enormous potential in several applications in the oil and cosmetics industry. The cultivation conditions for obtaining this bioproduct, however, still require attention, as, for example, parameters related to oxygen supply and consumption. In this study, different volumetric oxygen transfer coefficient (KLa) levels (0–11.56 h−1) were tested in bench-scale bioreactor for surfactin biosurfactant production by Bacillus velezensis H2O-1, using induced surface aeration. While conditions close to anaerobiosis showed insignificant production of surfactin, an intermediated KLa condition (4.24 h−1) generated the best surfactin concentration (579.6 mg L−1), with a volumetric productivity of 11.9 mg L−1 h−1. These results showed that the oxygen demand to produce surfactin is not high, being possible to use induced surface aeration strategy in bioreactors, minimizing foam formation. In addition, in all KLa conditions tested, surfactin homologues C14 and C15 had higher relative abundance. Nevertheless, the KLa parameter seems to have had minimal influence on affecting the relative abundances of surfactin homologues produced. Particularly noteworthy in this study is the possibility of producing surfactin using a low-cost and scale-up feasible aeration strategy, unlike the foam collection strategies developed in other studies to obtain this bioproduct.








Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
de Araujo LV et al (2016) Rhamnolipid and surfactin: Anti-adhesion/antibiofilm and antimicrobial effects. Food Control 63:171–178. https://doi.org/10.1016/j.foodcont.2015.11.036
Irorere VU, Tripathi L, Marchant R, McClean S, Banat IM (2017) Microbial rhamnolipid production: a critical re-evaluation of published data and suggested future publication criteria. Appl Microbiol Biotechnol 101(10):3941–3951. https://doi.org/10.1007/s00253-017-8262-0
Ongena M et al (2007) Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environ Microbiol 9(4):1084–1090
Malfanova N, Franzil L, Lugtenberg B, Chebotar V, Ongena M (2012) Cyclic lipopeptide profile of the plant-beneficial endophytic bacterium Bacillus subtilis HC8. Arch Microbiol 194(11):893–899. https://doi.org/10.1007/s00203-012-0823-0
Mnif I, Ghribi D (2015) Review lipopeptides biosurfactants: mean classes and new insights for industrial, biomedical, and environmental applications. PeptideScience 104:3. https://doi.org/10.1002/bip.22630
Hathout Y, Ho Y, Ryzhov V, Demirev P, Fenselau C (2000) Kurstakins : a new class of lipopeptides isolated from bacillus thuringiensis. J Nat Prod 63:1492–1496. https://doi.org/10.1021/np000169q
Velkov T, Roberts KD, Nation RL, Thompson PE, Li J (2014) Pharmacology of polymyxins: new insights into an ‘old’ class of antibiotics. Future Microbiol 8(6):1–20. https://doi.org/10.2217/fmb.13.39.Pharmacology
Coutte F et al (2017) Microbial lipopeptide production and purification bioprocesses, current progress and future challenges. Biotechnol J 12(7):1–10. https://doi.org/10.1002/biot.201600566
Théatre A et al (2021) The surfactin-like lipopeptides from bacillus spp.: natural biodiversity and synthetic biology for a broader application range. Bioeng. Biotechnol, Front. https://doi.org/10.3389/fbioe.2021.623701
Yeh M, Wei Y, Chang J (2006) Bioreactor design for enhanced carrier-assisted surfactin production with Bacillus subtilis. Process Biochem 41:1799–1805. https://doi.org/10.1016/j.procbio.2006.03.027
Chen C-Y, Baker SC, Darton RC (2006) Batch production of biosurfactant with foam fractionation. J Chem Technol Biotechnol 81:1923–1931
Nazareth TC, Zanutto CP, Maass D, Ulson De Souza AA, Ulson De Souza SMDAG (2021) Impact of oxygen supply on surfactin biosynthesis using brewery waste as substrate. J Environ Chem Eng 9:4. https://doi.org/10.1016/j.jece.2021.105372
Guez JS, Vassaux A, Larroche C, Jacques P, Coutte F (2021) New continuous process for the production of lipopeptide biosurfactants in foam overflowing bioreactor. Bioeng Biotechnol, Front. https://doi.org/10.3389/fbioe.2021.678469
Biniarz P, Henkel M, Hausmann R, Łukaszewicz M (2020) Development of a bioprocess for the production of cyclic lipopeptides pseudofactins with efficient purification from collected foam. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2020.565619
dos Santos LFM, Coutte F, Ravallec R, Dhulster P, Tournier-Couturier L, Jacques P (2016) An improvement of surfactin production by B. subtilis BBG131 using design of experiments in microbioreactors and continuous process in bubbleless membrane bioreactor. Bioresour Technol 218:944–952. https://doi.org/10.1016/j.biortech.2016.07.053
Coutte F, Lecouturier D, Leclère V, Béchet M, Jacques P, Dhulster P (2013) New integrated bioprocess for the continuous production, extraction and purification of lipopeptides produced by Bacillus subtilis in membrane bioreactor. Process Biochem 48(1):25–32
Berth A, Lecouturier D, Loubiere K, Dhulster P, Delaplace G (2019) Modelling and optimisation of gas-liquid mass transfer in a microporous hollow fiber membrane aerated bioreactor used to produce surfactin. Biochem Eng J 145:109–119. https://doi.org/10.1016/j.bej.2018.10.029
Sun D et al (2019) Effect of media and fermentation conditions on surfactin and iturin homologues produced by Bacillus natto NT-6: LC–MS analysis. AMB Express 9:1. https://doi.org/10.1186/s13568-019-0845-y
Bartal A et al (2018) Effects of different cultivation parameters on the production of surfactin variants by a Bacillus subtilis strain. Molecules 23:2–14
Jokari S, Rashedi H, Amoabediny GH, Yazdian F, Rezvani M, Hatamian Zarmi AS (2012) Effect of aeration rate on biosurfactin production in a miniaturized bioreactor. Int J Environ Res 6(3):627–634
Hoffmann M et al (2020) Towards the anaerobic production of surfactin using Bacillus subtilis. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2020.554903
Vigneshwaran C, Vasantharaj K, Krishnanand N, Sivasubramanian V (2021) “Production optimization, purification and characterization of lipopeptide biosurfactant obtained from Brevibacillus sp. AVN13. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2020.104867
Price NPJ, Rooney AP, Swezey JL, Perry E, Cohan FM (2007) Mass spectrometric analysis of lipopeptides from Bacillus strains isolated from diverse geographical locations. Microbiol Lett 271:83–89. https://doi.org/10.1111/j.1574-6968.2007.00702.x
Chen Y, Liu SA, Mou H, Ma Y, Li M, Hu X (2017) Characterization of lipopeptide biosurfactants produced by Bacillus licheniformis MB01 from marine sediments. Front Microbiol. https://doi.org/10.3389/fmicb.2017.00871
Shiau C (1995) Oxygen transfer in bubble and bubbleless aeration sistems. Doctor of philosophy thesis, department of civil and mining engineering, university of wollongong. https://ro.uow.edu.au/theses/1255
Korenblum E et al (2005) Production of antimicrobial substances by Bacillus subtilis LFE-1, B. firmus H2O–1 and B. licheniformis T6–5 isolated from an oil reservoir in Brazil. J Appl Microbiol 98(3):667–675. https://doi.org/10.1111/j.1365-2672.2004.02518.x
Korenblum E et al (2012) Purification and characterization of a surfactin-like molecule produced by Bacillus sp. H2O–1 and its antagonistic effect against sulfate reducing bacteria. BMC Microbiol. https://doi.org/10.1090/tran/7225
Guimarães CR et al (2019) Surfactin from Bacillus velezensis H2O–1: production and physicochemical characterization for postsalt applications. J Surfactants Deterg 22(3):451–462. https://doi.org/10.1002/jsde.12250
Guimarães CR (2015) Avaliação da produção de surfactina- like por Bacillus sp. H2O–1. Master of science thesis, Universidade Federal do Rio de Janeiro
Dunn IJ, Einsele A (1975) Oxygen transfer coefficients by the dynamic method. J Appl Chem Biotechnol 25(9):707–720
Sousa M, Melo VMM, Rodrigues S, Sant’ana HB, Goncalves LRB (2012) Screening of biosurfactant-producing Bacillus strains using glycerol from the biodiesel synthesis as main carbon source. Bioprocess Biosyst Eng 35(6):897–906. https://doi.org/10.1007/s00449-011-0674-0
Song B, Springer J (1996) Determination of interfacial tension from the profile of a pendant drop using computer-aided image processing: 2. Exp J Coll Inter Sci 184(1):77–91. https://doi.org/10.1006/jcis.1996.0598
Sheppard JD, Mulligan CN (1987) The production of surfactin by Bacillus subtilis grown on peat hydrolysate. Appl Microbiol Biotechnol 27(2):110–116. https://doi.org/10.1007/BF00251931
Liu X, Haddad NIA, Yang S, Mu B (2007) Structural characterization of eight cyclic lipopeptides produced by Ba- cillus subtilis HSO121. Protein Pept Lett 14:766–773
Strohalm M, Kavan D, Novák P, Volný M, Havlíček V (2010) mMass 3: a cross-platform software environment for precise analysis of mass spectrometric data. Anal Chem 82:4648–4651
Sarwar A, Corretto E, Aleti G, Abaidullah M, Sessitsch A, Hafeez FY (2017) Qualitative analysis of biosurfactants from Bacillus species exhibiting antifungal activity. PLoS ONE 13(6):1–15
Gu Q et al (2017) Bacillomycin D produced by bacillus amyloliquefaciens is involved in the antagonistic Interaction with the Plant-Pathogenic Fungus Fusarium graminearum. Appl Enviromental Microbiol 83(19):1–17
Kaisermann C (2017) Bioprocess intensification of surfactin production. Doctor of philosophy thesis. School of chemical engineering and analytical science university of manchester. https://www.research.manchester.ac.uk/portal/files/60829897/FULL_TEXT.PDF
Freire DA, Simonelli G, de Assis DJ, Druzian JI, Kluhpiuho A, Lobato DCL (2020) Surfactin production using papaya peel aqueous extract as substrate and its application for iron adsorption. Res Soc Dev 9(7):1–26
Kaneda T (1991) Iso- and anteiso-fatty acids in bacteria: Biosynthesis, function, and taxonomic significance. Microbiol Rev 55(2):288–302. https://doi.org/10.1128/mmbr.55.2.288-302.1991
Liu JF, Yang J, Yang SZ, Ye RQ, Mu BZ (2012) Effects of different amino acids in culture media on surfactin variants produced by Bacillus subtilis TD7. Appl Biochem Biotechnol 166(8):2091–2100. https://doi.org/10.1007/s12010-012-9636-5
Kracht M, Rokos H, Özel M, Kowall M, Pauli G, Vater J (1999) Antiviral and hemolytic activities of surfactin isoforms and their methyl ester derivatives. J Antibiot (Tokyo) 52(7):613–619. https://doi.org/10.7164/antibiotics.52.613
Assié LK et al (2002) Insecticide activity of surfactins and iturins from a biopesticide Bacillus subtilis Cohn (S499 strain). Meded Rijksuniv Gent Fak Landbouwkd Toegep Biol Wet 67(3):647–655
Jourdan E et al (2009) Insights into the defense-related events occurring in plant cells following perception of surfactin-type lipopeptide from Bacillus subtilis. Mol Plant Microbe Interact 22(4):456–468. https://doi.org/10.1094/MPMI-22-4-0456
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest in the publication.
Ethics approval
Not applicable.
Consent to participate
All authors consent to participate.
Consent for publication
All authors consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Paula Vieira de Castro, R., Alves Lima Rocha, V., Cezar Fernandes da Silva, M. et al. New insight into the role of oxygen supply for surfactin production in bench-scale bioreactors using induced surface aeration. Bioprocess Biosyst Eng 45, 2031–2041 (2022). https://doi.org/10.1007/s00449-022-02807-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-022-02807-8