Abstract
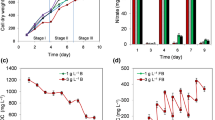
In this study, a 96-hole air-flowing device (96HAFD) was established for high-throughput screening of three mutant Chlorella strains under air aeration. 96HAFD was first tested for the confirmation of homogeneous air aeration cultivation environment at 1.2 L min−1 for algal screening based on the results of t test (p < 0.05) in the verification of consistency experiment. Then the data of dynamic growth characteristics of three mutant Chlorella strains indicated the good agreement in three screening devices including 96HAFD, flask and tube air-flowing cultivation devices by linear regression analysis between the 96HAFD and tube (R2 = 0.9904), 96HAFD and flask (R2 = 0.9904). At last, the 96HAFD was verified more efficient and reliable in fast screening single algal colony strains when compared with flask and tube air-flowing cultivation devices, because 96HAFD was confirmed have better performances in adaptation to the aeration cultivation circumstance and growing faster in a short period, in addition, 96HAFD had the less percentage of water loss per day (0.11%) than that of flask aeration device (2–3%) and tube aeration device (5–6.5%), which reduced negative effect caused by the water evaporation in the aeration cultivation to make the whole growing system more stable.






Similar content being viewed by others
References
Anandarajah K, Mahendraperumal G, Sommerfeld M, Hu Q (2012) Characterization of microalga Nannochloropsis sp. mutants for improved production of biofuels. Appl Energy 96:371–377
Catalado DA, Hanson M, Schrader LE, Yong VL (1975) Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun soil Sci Plant Anal 6(1):71–80
Cheng J, Huang Y, Feng J, Sun J, Zhou JH, Cen KF (2013) Mutate Chlorella sp. by nuclear irradiation to fix high concentrations of CO2. Biores Technol 136:496–501
De Morais MG, Costa JAV (2007) Isolation and selection of microalgae from coal fired thermoelectric power plant for biofixation of carbon dioxide. Energy Convers Manage 41:633–646
Guillard RRL (1973) Methods for microflagellates and nanoplankton. Handbook of phycological methods. Cambridge University Press, Cambridge UK, pp 80–81
Han W, Li CY, Miao XL (2012) A novel miniature culture system to screen CO2-sequestering microalgae. Energies 5:4372–4389
Heo J, Cho DH, Ramanan R (2015) PhotoBiobox: a tablet sized, low-cost, high throughput photobioreactor for microalgal screening and culture optimization for growth, lipid content and CO2 sequestration. Biochem Eng J 103:193–197
Hoshida H, Ohira T, Minematsu A, Akada R, Nishizawa Y (2005) Accumulation of eicosapentaenoic acid in Nannochloropsis sp. in response to elevated CO2 concentrations. J Appl Phycol 17:29–34
Huang GH, Chen F, Kuang YL, He H, Qin A (2016) Current techniques of growing algae using flue gas from exhaust gas industry: a review. Appl Biochem Biotechnol 178:1220–1238
Huang GH, Li T, Chen F, He H, Kuang YL (2016) High concentration CO2 sequestration by using microalgae in staged cultivation. Environ Prog Sustain Energy 35(6):1862–1867
Kao CY, Chiu SY, Huang TT, Dai L, Hsu LK, Lin CS (2012) ability of a mutant strain of the microalga Chlorella sp. to capture carbon dioxide for biogas upgrading. Appl Energy 93:176–183
Lee Y, Tay HS (1991) High CO2 partial pressure depresses productivity and bioenergetics growth yield of Chlorella pyrenoidosa culture. J Appl Phycol 3:95–101
Lukavsky J (1992) the evaluation of algal growth-potential (AGP) and toxicity of water by miniaturized growth bioassay. Water Res 26:1409–1413
Ma YB, Wang ZY, Zhu M, Yu CJ, Cao YP, Zhang DY, Zhou GK (2013) Increased lipid productivity and TAG content in Nannochloropsis by heavy-ion irradiation mutagenesis. Biores Technol 136:360–367
Negoro M, Shioji N, Miyamoto K (1991) Growth of microalgae in high CO2 gas and effects of SOX and NOX. Appl Biochem Biotechnol 28–9:877–886
Qi F, Pei H, Hu W, Mu R, Zhang S (2016) Characterization of a microalgal mutant for CO2 biofixation and biofuel production. Energy Convers Manage 122:344–349
Qi F, Wu DJ, Mu RM, Zhang S, Xu XY (2018) Characterization of a microalgal Uv mutant for CO2 biofixation and biomass production. Biomed Res Int 2018:1–8
Rodolfi L, Zittelli GC, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR (2009) Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102:100–112
Rojickova R, Dvorakova D, Marsalek B (1998) The use of miniaturized algal bioassays in comparison to the standard flask assay. Environ Toxicol Water Qual 13:235–241
Singh SP, Singh P (2014) Effect of CO2 concentration on algal growth: a review. Renew Sustain Energy Rev 38:172–179
Song MM, Pei HY, Hu WR, Ma GX (2013) Evaluation of the potential of 10 microalgal strains for biodiesel production. Biores Technol 141:245–251
Sung K-D, Lee J-S, Shin C-S (1999) CO2 fixation by Chlorella sp. KR-1 and its cultural characteristics. Bioresour Technol 68:269–273
Wang WL, Wei TT, Fan JH, Yi J, Li YG, Wan MX, Wang J, Bai WM (2018) Repeated mutagenic effects of 60CO-γ irradiation coupled with high-throughput screening improves lipid accumulation in mutant strains of the microalgae Chlorella pyrenoidasa as a feedstock for bioenergy. Algal Res 33:71–77
Wobbe L, Remacle C (2014) Improving the sunlight-to-biomass conversion efficiency in microalgal biofactories. J Biotechnol 201:28–42
Xu Z, Wang YJ, Chen YC, Spalding MH, Dong L (2017) Microfluidic chip for automated screening of carbon dioxide conditions for automated screening of carbon dioxide conditions for microalgal cell growth. Biomicrofluidics 11(6):064104
Yue L, Chen W (2005) Isolation and determination of cultural characteristics of a new highly CO2 tolerant fresh water microalgae. Energy Convers Manage 46:1868–1876
Acknowledgements
This project was financially supported by the Fundamental Research Funds for the Central Universities (2015XKMS041)
Funding
Fundamental Research Funds for the Central Universities, 2015XKMS041.
Author information
Authors and Affiliations
Contributions
Huang G H designed research; Han YY performed research. Huang and Han contributed reagents and analytic tools; Huang and Han analyzed data, Huang wrote the paper; All authors read and approved the manuscript. Authors agreement to authorship and submission of the manuscript for peer review.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Informed consent, human/animal rights
No conflicts, informed consent, human or animal rights applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, G., Han, Y., Li, W. et al. Rapid screening of microalgae by a 96-hole air-flowing device. Bioprocess Biosyst Eng 45, 943–953 (2022). https://doi.org/10.1007/s00449-022-02714-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-022-02714-y