Abstract
Global demand for poultry and associated feed are projected to double over the next 30 years. Insect meal is a sustainable alternative to traditional feeds when produced on low-value high-volume agricultural byproducts. Black soldier fly (BSF) larvae (Hermetia illucens L.) are high in protein and contain methionine, an essential amino acid that is critical to poultry health. BSF larvae can be grown on many organic residues, however, larvae growth and quality vary based on feedstock and cultivation processes. Experiments were completed to monitor temporal changes in BSF larvae growth and composition using almond hulls as a growth substrate under batch and semi-batch processes and with varying substrate carbon to nitrogen ratio (C/N). A logistic kinetic growth model was developed to predict larval biomass and methionine accumulations during batch production. Estimated ranges of model parameters for larvae maximum specific growth rate and carrying capacity were 0.017–0.021 h−1 and 9.7–10.7 g larvae kg−1 hulls dry weight, respectively. Methionine content in larvae increased from 11.1 to 17.1 g kg−1 dry weight over a 30-day batch incubation period. Larvae-specific growth and yield increased by 168% and 268%, respectively, when cultivated in a semi-batch compared to a batch process. Increasing C/N ratio from 26 to 40 increased density of methionine content in larvae per unit feedstock by 25%. The findings demonstrate a logistic model can predict larvae biomass accumulation, harvest time can achieve specific methionine contents, and a semi-batch process is more favorable for larvae biomass accumulation compared to a batch process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The human global population is projected to reach 9.5 billion by 2050 which will put increasing demands on food production [1]. Finding methods that increase food production on existing natural resources is critical for addressing growing needs for nutritious food. Producing insect biomass requires less land and resources compared to the production of other protein sources. For example, the land required to produce 1 kg of beef, soy, mealworm and black soldier fly (BSF) larvae is 12.5, 3.5, 0.60 and 0.22 square meters, respectively [2,3,4,5]. BSF are desirable for insect farming compared to other insects, because they have a relatively fast life cycle, they are not a vector for disease, they exhibit “self-harvesting” behavior, and they have a favorable composition to meet nutritional requirements of humans and animals [6, 7]. BSF larvae are high in protein, fat, amino acids, essential fatty acids and minerals [8, 9]. Many studies have demonstrated BSF larvae meal is comparable to soy and fishmeal and can be substituted into diets for poultry, fish and humans [10,11,12]. BSF larvae can also grow on a variety of organic substrates and low-value, high-volume agricultural residues, and could play a role in closing the loop between organic waste recycling and food production.
One potential insect growth substrate that is abundant in California is almond hulls, which are derived from the processing of almonds. The almond kernel is surrounded by a shell and an outer hull. In 2018, the California almond industry produced approximately 1 million metric tons of almond kernels and over 2 million metric tons of almond hulls [13]. While almond hulls are a suitable feedstock for BSF larvae cultivation, larvae growth and composition vary with growth conditions [14, 15]. For example, larvae harvest dry weight increased from 5.4 to 9.2 mg larvae−1 when carbon to nitrogen (C/N) ratio of almond hull feedstock was decreased from 49 to 16 by amending hulls with urea and incubation at 28 °C [15]. Also, methionine content in harvested larvae increased from 4.15 to 4.63 g kg−1 dry weight when temperature was decreased from 32 to 28 °C and C/N was adjusted to 16 [15]. It is important to understand the impacts of cultivation environment on larvae biomass accumulation and composition to enable the consistent production of BSFL biomass for animal feeds and other products [7].
Kinetic models are one way to predict BSF larvae biomass accumulation in a system. For example, a study confirmed the logistic function could be used to compute growth rates for BSF larvae by validating data sets from a variety of experiments including variables of temperature, feed quality, feeding rate, feed moisture content and airflow rate [16]. The Verhulst logistic growth model was used to find the specific growth rates of BSF larvae grown on mixtures of degassed sludge, industrial organic waste and commercial chicken feed to estimate costs of growth, maintenance metabolism, rates of feed assimilation and net growth efficiency [17]. Another study monitored the daily growth of BSF larvae reared on ten different substrates and found the Richards model was the best fit growth model and that larvae followed an S-shaped growth curve [18]. The logistic growth model has been used to describe BSF larvae dry weight over time from day 0 after inoculation until reaching prepupae and maximal weight [17, 19]. Furthermore, prior observations of BSF larvae growth as a function of time indicate growth follows an exponential growth pattern and reaches a stationary phase during pre-pupation [20]. These findings suggest a logistic model could be used to predict BSF larvae growth on almond hulls for improving system performance for large-scale production. Furthermore, accounting for nutrient accumulation in a prediction model would significantly benefit animal feeding industries.
The main objectives of this research were to (1) develop a kinetic model for BSF larvae growth to facilitate the prediction of larvae biomass and methionine accumulation using almond hulls as substrate, (2) measure the dynamic changes in nutrient content in BSF larvae to provide insight into how harvest time could be managed to overcome varying nutrient content in larvae, (3) and test the impact of batch (single substrate feeding) versus semi-batch (multiple substrate feedings) cultivation on larvae and methionine production. The results demonstrate that BSF larvae and methionine accumulation follow a logistic model under specific environmental conditions, altering harvest time is one way to achieve a specific methionine content in larvae, and producing larvae in a semi-batch process enhances insect biomass and methionine production compared to batch production.
Materials and methods
Acquisition and processing of almond hull feedstock
A Monterey variety of almond hulls and shells was obtained from a processor in Buttonwillow, CA from the 2017 harvest and used as larvae cultivation feedstock. The hulls were ground using a hammer mill with a 6.35 mm screen and then stored in airtight plastic bags. The as-received moisture content of the material was 18.2% (dry basis). Composition analyses were done by UC Davis Analytical Laboratories (Davis, CA). Total nitrogen and carbon were measured according to AOAC 972.43, through the flash combustion method using thermal conductivity/IR detection (LECO FP-528 and TruSpec CN Analyzers) [21]. Total crude protein was calculated from the nitrogen content with a protein factor of 6.25 [22]. Fat was measured using the Randall modification of the standard Soxhlet extraction [23]. Calcium was measured using nitric acid/hydrogen peroxide microwave digestion and determination by Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES) [24, 25]. Total sugar was measured by enzymatically hydrolyzing at 55 °C with amyloglucosidase for 12 h and analyzed by high performance liquid chromatography (HPLC) with mass selective detection [26]. Total starch was calculated from the total and free glucose [26]. Neutral detergent fiber was measured using Association of Official Agricultural Chemists (AOAC) 2002–04 [23]. The acid detergent fiber and acid detergent lignin were measured using AOAC 973.18 [27]. The material was reported to have 8.6 g kg−1 total nitrogen, 411.1 g kg−1 total carbon, 53.8 g kg−1 protein, 20.8 g kg−1 fat, 2.8 g kg−1 calcium, 100.0 g kg−1 total sugar, 5.50 g kg−1 total starch, 348.5 g kg−1 acid detergent fiber, 409.0 g kg−1 neutral detergent fiber, and 67.8 g kg−1 acid detergent lignin.
BSF larvae rearing
Larvae rearing methods were conducted as described in detail elsewhere using eggs supplied by Trevor Fowles (Department of Entomology, University of California, Davis, USA) [14]. Larvae were reared on chicken feed (Purina Premium Poultry Feed Layena Crumbles, Purina Animal Nutrition LLC, Shoreview, MN) at a moisture content of 500 g kg−1 wet basis and incubated at 28 °C. 7–9-day-old larvae were used in the experiments. In the first experiment, larvae were separated from feed using 1 and 2 mm sieves. To have consistent larvae size in the second experiment, larvae were manually separated from feed based on size. Prior to inoculation onto hulls, samples of larvae were collected for moisture content measurement and average dry weight per larvae.
Feedstock preparation and incubations
Feedstock was amended with distilled water and nitrogen prior to incubation. Urea (Fisher Scientific Company LLC, Hampton, NH) was added to distilled water to achieve target carbon to nitrogen (C/N) ratios in the hull mixtures. Urea was used as a model nitrogen source in these studies rather than an organic waste source of nitrogen, because urea could be obtained consistently and distributed uniformly throughout the hulls. Adjusting C/N of hulls using urea was found to significantly impact larvae growth in prior studies [15]. From each mix, three random samples were collected to measure pH and moisture content prior to larvae inoculation. Table 1 lists the C/N ratios, moisture content, initial pH of hulls, inoculation density of larvae, average number of larvae per bioreactor, average initial larvae size, aeration rate and incubation temperature for each experiment. Larvae were cultivated in 1500 mL bioreactors as described in detail elsewhere [14, 15]. Humidified air was provided to the bioreactors by bubbling house air through distilled water and metered to each bioreactor with polycarbonate rotameters (5–50 mL min−1, Dwyer Instruments, Michigan City, IN). Clear poly tubing was secured to the inlet and joined with a 6.4 mm diameter black porous soaker drip line to ensure uniform aeration through the bed of feedstock.
The first experiment was designed to measure larvae biomass and methionine accumulation as a function of time in batch culture. One bioreactor was harvested every 48 h for a total of 15 bioreactors (time points over 30 days). The feeding rate was calculated by dividing the initial hulls dry weight by the number of larvae and incubation period and was approximately 67 mg dry/larvae/day over a 30-day cultivation period and 100 mg dry/larvae/day over a 14-day cultivation period (the latter used in the model validation). Larvae biomass and methionine and cystine contents were measured at each time point. Data from this experiment were used to develop the kinetic model and inform the hull feeding amount and the time of feeding for the second experiment.
The second experiment was designed to determine the impact of semi-batch cultivation and C/N ratio on larvae growth and composition. The C/N ratio of the initial feedstock fed to all bioreactors was 26. For the second feeding, which occurred at 150 h, the feedstock was adjusted to achieve four different C/N ratios (26, 33, 40 and 47) prior to feeding. The time of the second feeding was determined based on the logistic growth period observed in the first experiment. One batch control treatment was fed all the hulls during the initial feeding with no additional hulls added throughout the cultivation period. This batch treatment was prepared to validate the kinetic parameters found from the first experiment and to compare results from batch versus semi-batch cultivation. The total amount of hulls fed for all treatments in this experiment (200 dry grams) was split evenly between the two feedings for a feeding rate of approximately 143 mg dry/larvae/day. Three replicate bioreactors were prepared for each treatment for a total of 15 bioreactors. The responses included specific larvae growth, average larvae harvest weight, larvae yield, hull consumption, methionine and cystine contents of harvested larvae (g amino acid kg−1 larvae), methionine accumulation (g larvae methionine content kg−1 hulls), and total methionine produced. The cultivation studies ran for 14 days. Temperature inside each bioreactor was monitored using iButton temperature loggers (Thermochron iButton DS1921G-F5#, Embedded Data Systems, Lawrenceburg, KY) wrapped in thermoplastic wrap (Parafilm M Wrapping Film, Fischer Scientific, Hampton, NH). Temperature data were logged every 15 min (Fig. S1).
Larvae harvest and analysis
At the end of the experiments, the contents of bioreactors were frozen at − 20 °C. Larvae were separated and counted at a later date; total larvae weight and numbers of larvae recovered per reactor were recorded. Larvae moisture content was measured for each treatment. Separated larvae were stored at − 20 °C and homogenized with an oscillating ball mill (MM400, Retsch Inc., Newtown, PA). The homogenized larvae were freeze dried (VirTis 50-SRC-5, SP Scientific, Warminster, PA) for 4 days. The methionine and cystine contents were measured at the UC Davis Genome Center, Molecular Structure Facility (Davis, CA, USA) using performic oxidation with hydrolysis [28].
Spent substrate analysis
At the end of the experiments, samples of spent hull substrate were analyzed for moisture content and pH using methods as previously described [28]. For both experiments, moisture content and pH were analyzed at the time of larvae harvest. For the first experiment, samples of hulls were freeze dried and methionine and cystine were measured using the same method described above for larvae.
Data analysis
Larvae biomass and methionine accumulation kinetics during batch production were modeled using the logistic equation (Eqs. 1 and 2):
where Xb = larvae biomass density (g larvae kg−1 hulls (dry weight)), fm = methionine content in larvae biomass (g larvae methionine g−1 larvae), Xm = methionine density (g larvae methionine kg−1 hulls (dry weight)), t = time (hr), Xmax,b = maximum larvae biomass density (g larvae kg−1 hulls (dry weight)), Xmax,m = maximum methionine density during batch growth (g larvae methionine kg−1 hulls (dry weight)), μmax,b = maximum specific larvae biomass accumulation rate (h−1), and μmax,m = maximum specific methionine accumulation rate (h−1). It was assumed that methionine content remained constant during the period of logistic growth. Larvae methionine accumulation differs from the larvae methionine content, such that the accumulation represents the total larvae methionine content per total feedstock, or the density of methionine content in the system. The logistic equations were integrated using an initial density of Xb = X0,b and Xm = X0,m at t = 0 to obtain the following forms (Eqs. 4 and 5):
Parameter estimates in Eqs. (4) and (5) were determined using experimental data and the nlinfit nonlinear regression function in MATLAB (MATLAB R2017b, MathWorks, Natick, MA).
The larvae yield and hull consumption were calculated as described in detail elsewhere [14]. For the second experiment, responses for yield, hull consumption, average larvae harvest dry weight, final larvae composition, methionine accumulation and total methionine produced were analyzed as a function of C/N ratio. Interactions between C/N ratio and feeding frequency was also investigated. Standard least squares regression analyses and Tukey’s HSD tests were performed using JMP-IN software (version Pro 12, SAS, Cary, NC). The significance level was set to 0.05.
Results
Batch process
Kinetic model development
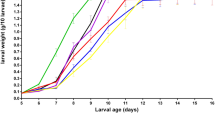
The logistic growth model fit larvae biomass accumulation data through the early pupa life stages for batch cultivation, shown in Fig. 1. The exponential growth phase was observed to be during the first 144 h of growth and the stationary phase to be between approximately 144–400 h of growth. Biomass data were used to estimate parameters in the logistic growth model (Table 2). The parameter estimates were 10.2 g larvae kg−1 hulls (dry weight) for \({X}_{\mathrm{max},b}\), and 0.019 h−1 for \({\mu }_{\mathrm{max},b}\). The root mean squared error was 4.4 g larvae kg−1 hulls indicating a meaningful fit between the model and the biological data.
Larvae growth on almond hulls in a batch process in Experiment 1. The points represent measured larvae biomass density for a single bioreactor with the approximate life cycle stage determined through visual inspection. The line represents the model fit of the logistic equation (Eq. 4)
Methionine accumulation data for batch cultivation of larvae are provided in Fig. 2. These data were used to estimate parameters in the logistic growth model (Table 2). The parameter estimates were 0.137 g larvae methionine kg−1 hulls (dry weight) for \({X}_{\mathrm{max},m}\) and 0.015 h−1 for \({\mu }_{\mathrm{max},m}\). The root mean squared error was 0.06 g larvae methionine kg−1 hulls indicating a meaningful fit between the model and the experimental data. The difference between \({\mu }_{\mathrm{max},b}\) and \({\mu }_{\mathrm{max},m}\) may have been due to the change in methionine content in larvae during batch growth (Fig. 3).
Methionine accumulation in harvested larvae biomass in Experiment 1. The points represent the measured methionine accumulation in harvested larvae for a single bioreactor and the line represents the model fit of the logistic equation (Eq. 5)
Validation of kinetic model
Batch cultivation data from the control treatment of the second experiment were used to validate the biomass accumulation model using Eq. (4). Estimates for Xmax,b and μmax,b (Table 2) and the initial condition for X0,b for the second experiment (1.9 g larvae kg−1 hulls (dry weight)), predicted the larvae biomass density to be 10.1 g larvae kg−1 hulls (dry weight) after 336 h of growth. The measured larvae biomass density in the control treatment in the second experiment at that time was 9.3 g larvae kg−1 hulls (dry weight) with a standard deviation of 1.2 g larvae kg−1 hulls (dry weight). The percent error was approximately 7.9% between the expected and experimental values.
Batch cultivation data from the second experiment were also used to validate the methionine accumulation model. Using Eq. 5, estimates for Xmax,m and μmax,m (Table 2) and the initial condition for X0,m for the second experiment [0.023 g larvae methionine kg−1 hulls (dry weight)], the predicted methionine density was 0.13 g larvae methionine kg−1 hulls (dry weight) after 336 h of growth. The measured methionine density in the control treatment of the second experiment was 0.10 g larvae methionine kg−1 hulls (dry weight) with a standard deviation of 0.018 g larvae methionine kg−1 hulls (dry weight). The percent error was approximately 23% between the expected and experimental values.
Changes in larvae composition over time
Methionine content in larvae ranged from 10.4 g kg−1 dry larvae to 17.1 g kg−1 dry larvae over the 30-day batch experiment (Fig. 3). Linear regression of larvae methionine content with respect to time showed a significant first-order relationship (Table 3, Fig. 3). Cystine content of harvested larvae averaged 8.5 g kg−1 dry larvae throughout the study (Fig. 3). There were no significant changes in harvested larvae cystine content with respect to time (P > 0.05).
Hull consumption and composition over time
Hull consumption ranged from 7 to 29% over the first 6 days of the experiment and averaged 24% for the remaining 24 days (Fig. 4). Linear regression of hull consumption data with respect to time for the first 6 days of cultivation showed a significant first-order relationship (Table 3). The pH of hulls increased from 4.72 to 7.92 over the first 8 days and averaged 8.16 for the remaining 22 days (Fig. 5). Hull methionine and cystine contents averaged 4.69 g kg−1 dry and 4.22 g kg−1 dry, respectively (Fig. 6), throughout the experiment with no significant trends at the beginning of the experiment.
Semi-batch process
Larvae growth
Increasing the number of feedings from one to two over the 14-day experiment significantly impacted the specific larvae growth, average larvae harvest weight and larvae yield (Table 4). Average specific larvae growth was 2.7 times higher when the feedings were increased from one to two, 2.68 g g−1 dry and 7.24 g g−1 dry, respectively. Average larvae harvest weight was 0.015 g dry larvae−1 and 0.033 g dry larvae−1 for one and two feedings, respectively, increasing by 2.2 times. Average larvae yield was 4.63 g g−1 dry and 14.96 g g−1 dry for one and two feedings, respectively, a 3.2 times increase. Varying the C/N ratio for the second feeding did not significantly impact the specific larvae growth, average larvae harvest weight or larvae yield (P > 0.05).
Larvae composition
Average methionine and cystine contents of harvested larvae were 11.6 ± 0.43 g kg−1 dry and 6.71 ± 0.63 g kg−1 dry, respectively, for the single feeding, and 10.8 ± 0.39 g kg−1 dry and 5.36 ± 0.31 g kg−1 dry, respectively, for all treatments with two feedings (Fig. 7). Increasing the number of feedings during the cultivation period decreased larvae cystine content nearly 1.3 times, from 6.71 g kg−1 dry to 5.36 g kg−1 dry. Increasing the number of feedings had a significant and negative first-order effect on larvae cystine content (P < 0.05, Table 5), but had no effect on methionine content.
Methionine and cystine contents in black soldier fly larvae grown on almond hulls in Experiment 2. Each point represents the average of bioreactor replicates. Three replicates for all treatments except two replicates for treatments with C/N of 26 and two feedings C/N of 47 due to mishandling of the samples prior to analysis
Methionine accumulation and production
Regression analysis revealed increasing C/N ratio had significant and positive first-order effects on methionine accumulation and methionine production (P < 0.05, Table 5). When the C/N ratio was increased from 26 to 40, the average methionine accumulation increased by 1.2 times, from 0.189 g kg−1 dry to 0.233 g kg−1 dry. This trend was also observed with total methionine production, which increased nearly 1.3 times (from 30 mg dry to 38 mg dry). Increasing the number of feedings from one to two increased average methionine accumulation by 1.8 times (from 0.104 g kg−1 dry to 0.189 g kg−1 dry) and increased average methionine production by 1.9 times (from 16 mg dry to 30 mg dry).
Hull consumption and pH
Average hull consumption was statistically different and approximately 1.8 times lower for the bioreactors with a single feeding of C/N 26 compared to treatments with two feedings of C/N ratios 33 and 47, ranging from 0.23 to 0.42 g g−1 dry, respectively (Table 4). Average pH of spent material varied between 7.86 and 8.55 and was not statistically different between treatments (P > 0.05).
Discussion
Kinetic growth model
The logistic model successfully predicted BSF larvae accumulation (Table 2). This was consistent upon analysis of data from prior reports (Fig. S2) which showed that BSF larvae follow a logistic curve and reach a stationary growth phase based on the transformation from early prepupa to late pupa (Fig. S2) and prior research [16, 29]. In the model validation, methionine accumulation was overpredicted. This may have been due to several factors. Although the environmental variables were consistent in both experiments using almond hulls as feedstock, the inoculation density, average larvae weight in the inoculum and number of larvae varied between the two experiments (Table 1). In the second experiment (C/N feeding), there were 43% fewer larvae per bioreactor which were double the initial average dry weight at time of inoculation compared to the first experiment (kinetic growth). The larvae in the inoculum of the second experiment were larger than the first experiment, suggesting they were farther along in development and may have reached the stationary phase sooner than larvae in the first experiment. The model does not factor in stage of growth or number of larvae and both were different between experiments which may have contributed to differences in methionine accumulation. A future model might consider stage of growth and number of larvae in the initial conditions.
To further investigate the logistic model and the quality of almond hulls as a feedstock, another data set from prior studies on the dynamic changes of nutrient composition throughout the BSF larvae life cycle was evaluated [29]. In these studies, larvae were cultivated on chicken feed and dry mass of individual BSF larvae was measured. Random sampling was performed at time of feedings on days 4, 6, 7, 9, 12 and 14. Upon examination of these prior data, it can be shown that larvae cultivated on chicken feed also followed a logistic growth curve and the exponential and stationary phases occurred in the BSF larvae life cycle regardless of resources and environment (Fig. S2). However, the kinetic parameters varied with the cultivation feedstock and environmental conditions. BSF larvae grown on chicken feed in a semi-batch operation had approximately 53% higher larvae growth rate (μmax) compared to BSF larvae grown on almond hulls in batch cultivation, however, the estimated carrying capacity (\({X}_{\mathrm{max}}\)) was approximately 4–18 times greater for larvae grown on almond hulls, depending on the total amount of chicken feed added throughout the experiment (Table 6).
It is well known that cultivation variables such as feedstock, larval density, feeding rate and environmental factors affect the BSF larvae development time and larvae weight. Variation in kinetic parameters using different feedstocks have been shown in prior studies, where BSF larvae had the lowest maximum absolute growth rate and delayed time to reach the maximum absolute growth rate in feedstocks with the lowest protein content [18]. Harnden and Tomberlin [30] reported BSF larvae cultivated on a pork diet took more time to complete larval development than larvae cultivated on beef and grain diets; approximately 23.1% and 139.7% longer, respectively. Barragan-Fonseca et al. [31] investigated the effect of dietary nutrition of feedstock and larval rearing density by mixing chicken feed and cellulose into three different ratios and feeding four different BSF larval densities. In all cases, those fed the diet highest in cellulose and lowest in protein (77% cellulose and 23% chicken feed), had an average 30% longer development time or did not complete larval development at all [31]. Myers et al. [32] reported that increasing the daily feeding rate by 61.4% increased BSF larvae prepupal weight by 34.8% and decreased larval development time by 14.9%. In the present batch cultivation study, BSF larvae were given all feedstock initially, whereas the prior study provided an additional five feedings over the course of 14 days. Increasing the number of feedings using almond hulls as feedstock would likely have a positive effect on the specific larvae growth rate based on results from fed-batch experiments.
Other environmental variables such as type of rearing environment, temperature, and moisture content varied between the present and prior studies and may have contributed to the varying kinetic parameters (Table 7). Temperature has been shown to be a critical parameter for larvae growth. For example, dry weight of larvae was 25% greater for larvae reared on almond hulls at 34 °C compared to 28 °C [15] and 27% greater for larvae reared on pork at 32.2 °C compared to 27.6 °C [30]. In another study, Tomberlin et al. [33] demonstrated temperature differences as little as 3 °C produced significant effects on larval development. In prior experiments using almond hulls as feedstock, average dry weight of larvae was 45% greater when moisture content was increased from 480 g kg−1 (wet basis) to 680 g kg−1 and a positive linear relationship was observed between moisture content and larvae weight [28]. Almond hulls contain more fibers than chicken feed and can hold more water and this additional water may have increased larvae growth. In other words, the 600 g kg−1 wet basis chicken feed may have had more free water available than 690 g kg−1 wet basis almond hulls.
In addition to cultivation and environmental variables, the time of harvest can further impact larvae composition including lipid, carbohydrate, amino acid and mineral content. In the present study, methionine content in harvested larvae increased by 54% reaching 17.1 g kg−1 at the end of 30 days. In a prior study by Liu et al. [29], the methionine content of black soldier fly increased by 71% after approximately 30 days of growth on chicken feed (Fig. S3). Similar to the present study, linear regression of larvae methionine content revealed significant first-order effects as larvae developed over time with a slope of 0.63 and p value of 0.0003. While the impact of cultivation feedstock on larvae composition is well known, our findings indicate that time of harvest can also be managed to optimize amino acid content such as methionine content.
Semi-batch cultivation
Several studies have determined the effect of feeding frequency on BSF larvae production. Diener et al. (2009) used chicken feed to cultivate BSF larvae at five daily feeding rates of 12.5, 25, 50, 100 and 200 mg/larvae/day and found that a daily feeding rate of 100 mg/larvae/day was optimal for conversion efficiency and substrate consumption [34]. Another study cultivated BSF larvae on rice straw byproduct at the same daily feeding rates of 12.5, 25, 50, 100 and 200 mg/larvae/day and found the larvae with the highest daily feeding rate of 200 mg/larvae/day yielded the highest prepupal dry weight but the lowest waste reduction efficiency [35]. A study that cultivated BSF larvae on human feces as a sanitation and waste treatment strategy found a single batch feeding resulted in an average of 38% higher prepupal weight compared to six feedings totaling the same amount as the single feeding [36]. In the present study, using the same amount of almond hulls but increasing the feedings from one to two, increased specific larvae growth and yield by approximately three times and average larvae harvest weight by two times. It was observed that the addition of feedstock resulted in an initial increase in temperature within the bioreactors. Prior studies with a similar feedstock demonstrated that increasing temperature from 28 to 34 °C had a significant and positive effect on larvae harvest dry weight, specific larvae growth and larvae yield [15]. For this reason, the increase in temperature associated with addition of feedstock may be one of the contributing factors to the increased growth. Another factor that likely played a role on larvae growth was the time of additional feedings during the logistic growth period. Future experiments could be done to determine optimal feedstock amount, number of feedings and time of feedings for BSF larvae grown on almond hulls.
There are very few studies that have directly investigated the effect of feedstock C/N ratio on the cultivation of larvae. In our previous research, we found that varying C/N ratio using a Pollinator variety of almond hulls as feedstock and urea as a model nitrogen source can affect BSF larvae growth and methionine content. While the direct mechanism of nitrogen amendment on larvae was not investigated in the current study, supplemented nitrogen is important for the growth of microorganisms and synthesis of enzymes necessary to breakdown lignocellulose in nitrogen poor substrates like almond hulls [15]. In the prior study, decreasing C/N ratio from 49 to 16 by increasing amendment of urea decreased larvae methionine by 11% and increased larvae specific growth by approximately 30% [15]. In the present study, a similar relationship was observed with methionine accumulation and total methionine production; where decreasing C/N from 40 to 26 decreased methionine accumulation and total methionine production by 25% and 26%, respectively. Another factor that may have contributed to differences observed in larvae growth is the almond hull type used in the cultivation studies. In another of our previous studies, we compared BSF larvae cultivation on six different almond hull samples. BSF-specific larvae growth (g g−1 dry) and harvest average larvae weight (g dry larvae−1) were highest for a Monterey variety and approximately 12% and 6.6% higher, respectively, than the average of the Pollinator varieties [37]. The study also reported Monterey hulls had the lowest C/N ratio of 42 compared to the other samples and was approximately 1.7 times lower than the Pollinator varieties, which averaged a C/N of 71 [37]. It is possible that the almond hull nitrogen in the current study using Monterey hulls (C/N of 47) had a higher bioavailability compared to the previous study that used Pollinator hulls. Also, since the C/N ratio was achieved by adding urea to the water in the hull mixture, it is possible nitrogen in urea was more accessible and, therefore, played an important role in the growth and yield of larvae.
Conclusion
The results demonstrate a logistic model can be used to estimate BSF larvae biomass accumulation when grown on almond hulls and chicken feed. Methionine content of BSF larvae produced on almond hulls showed a linear increasing trend with cultivation time. Increasing the number of feedings from one to two increased the growth, weight and yield of BSF larvae grown on almond hulls. Methionine accumulation and methionine production were significantly impacted by C/N ratio. These results reinforce that careful management of environmental conditions and feedstock need to be considered to optimize larvae production and quality.
References
DESA U (2019) World Population Prospects 2019. United Nations. Department of Economic and Social Affairs. World Population Prospects 2019
USDA (2018) National Agriculture Statistics Service. Statistics by subject
Ramos-Elorduy JGE, Hernández AR, Pino JM (2002) Use of Tenebrio molitor (Coleoptera: Tenebrionidae) to recycle organic wastes and as feed for broiler chickens. J Econ Entomol 95(1):214–220
Lundy M, Parrella M (2015) Crickets are not a free lunch: protein capture from scalable organic side-streams via high-density populations of Acheta domesticus. PLoS ONE 10:e0118785
Spranghers T et al (2017) Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J Sci Food Agric 97(8):2594–2600
Čičková H et al (2015) The use of fly larvae for organic waste treatment. Waste Manag 35(Supplement C):68–80
Barragan Fonseca K, Dicke M, van Loon J (2017) Nutritional value of the black soldier fly (Hermetia illucens L.) and its suitability as animal feed. J Insects Food Feed 3:105–120
Bußler S et al (2016) Recovery and techno-functionality of flours and proteins from two edible insect species: meal worm (Tenebrio molitor) and black soldier fly (Hermetia illucens) larvae. Heliyon 2(12):e00218
Smetana S et al (2016) Sustainability of insect use for feed and food: life cycle assessment perspective. J Clean Prod 137(Supplement C):741–751
Al-Qazzaz MFA et al (2016) Effect of using insect larvae meal as a complete protein source on quality and productivity characteristics of laying hens. Revista Brasileira de Zootecnia 45:518–523
Kroeckel S et al (2012) When a turbot catches a fly: evaluation of a pre-pupae meal of the Black Soldier Fly (Hermetia illucens) as fish meal substitute—growth performance and chitin degradation in juvenile turbot (Psetta maxima). Aquaculture 364–365:345–352
Wang Y-S, Shelomi M (2017) Review of black soldier fly (Hermetia illucens) as animal feed and human food. Foods 6:91
ABC (2018) Almond Tree fruit weight: 2017/2018 crop year. Almond Board of California: Supplement to July 2018 Position Report
Palma L et al (2018) Cultivation of black soldier fly larvae on almond byproducts: impacts of aeration and moisture on larvae growth and composition. J Sci Food Agric 98(15):5893–5900
Palma L et al (2019) Managing high fiber food waste for the cultivation of black soldier fly larvae. NPJ Sci Food 3(1):15
Padmanabha M et al (2020) A comprehensive dynamic growth and development model of Hermetia illucens larvae. PLoS ONE 15(9):e0239084
Laganaro M, Bahrndorff S, Eriksen NT (2021) Growth and metabolic performance of black soldier fly larvae grown on low and high-quality substrates. Waste Manag 121:198–205
Sripontan Y et al (2020) Modeling the growth of black soldier fly Hermetia illucens (Diptera: Stratiomyidae): an approach to evaluate diet quality. J Econ Entomol 113(2):742–751
Bekker NS et al (2021) Impact of substrate moisture content on growth and metabolic performance of black soldier fly larvae. Waste Manag 127:73–79
Maino JL, Kearney MR (1819) Testing mechanistic models of growth in insects. Proc R Soc B Biol Sci 2015(282):20151973
AOAC (1997) Official method 972.43, microchemical determination of carbon, hydrogen, and nitrogen, automated method. In: official methods of analysis of AOAC International. A. International, Editor, Arlington, pp 5–6
AOAC (20060 Official method 990.03: protein (crude) in animal feed, combustion method. Official Methods of Analysis of AOAC International. AOAC International, Arlington, pp 30–31
AOAC (2006) Official method 2002–04, amylase-treated neutral detergent fiber in feeds: using refluxing in beakers or crucibles. Official methods of analysis of AOAC International, chapter 4. AOAC International, Arlington
Meyer GA (1992) An overview of analysis by inductively coupled plasma-atomic emission spectrometry. In: Montaser A, Golightly DW (eds) Inductively coupled plasmas in analytical atomic spectrometry. VCH Publishers, New York
Sah RN, Miller RO (1992) Spontaneous reaction for acid dissolution of biological tissues in closed vessels. Anal Chem 64:230–233
Smith D (1969) Removing and analyzing total nonstructural carbohydrates from plant tissue. Wisconsin Agriculture Experiment Station Research Report 41
AOAC (1997) Official method 973.18: fiber (acid detergent) and lignin in animal feed. Official methods of analysis of AOAC international, chapter 4. AOAC International, Arlington
AOAC (1997) Official method 994.12. Amino acids in feeds. Alternatie II, performic acid oxidation with acid hydrolysis—hydrobromic acid method. In: Official methods of analysis of AOAC International. Association of Official Analytical Chemists, Gaithersburg
Liu X et al (2017) Dynamic changes of nutrient composition throughout the entire life cycle of black soldier fly. PLoS ONE 12(8):e0182601
Harnden LM, Tomberlin JK (2016) Effects of temperature and diet on black soldier fly, Hermetia illucens (L.) (Diptera: Stratiomyidae), development. Forensic Sci Int 266:109–116
Barragan-Fonseca KB, Dicke M, van Loon JJA (2018) Influence of larval density and dietary nutrient concentration on performance, body protein, and fat contents of black soldier fly larvae (Hermetia illucens). Entomol Exp Appl 166(9):761–770
Myers HM et al (2008) Development of black soldier fly (Diptera: Stratiomyidae) larvae fed dairy manure. Environ Entomol 37(1):11–15
Tomberlin J, Adler P, Myers H (2009) Development of the black soldier fly (Diptera: Stratiomyidae) in relation to temperature. Environ Entomol 38:930–934
Diener S, Zurbrügg C, Tockner K (2009) Conversion of organic material by black soldier fly larvae: establishing optimal feeding rates. Waste Manag Res 27(6):603–610
Manurung R et al (2016) Bioconversion of Rice straw waste by black soldier fly larvae (Hermetia illucens L.): optimal feed rate for biomass production. J Entomol Zool Stud 4(4):1036–1041
Banks I, Gibson W, Cameron M (2014) Growth rates of black soldier fly larvae fed on fresh human faeces and their implication for improving sanitation. Trop Med Int Health TM IH 19:14–22
Palma L et al (2020) Almond by-product composition impacts the rearing of black soldier fly larvae and quality of the spent substrate as a soil amendment. J Sci Food Agric 100(12):4618–4626
Acknowledgements
This work was supported by the Almond Board of California [project 17-VandergheynstJ-COC-01], the National Institute of Food and Agriculture [project CA-D-BAE-2228-RR], and a gift from the Methionine Task Force. The sponsors had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Miner, L.P., Fernandez-Bayo, J., Putri, F. et al. Predicting black soldier fly larvae biomass and methionine accumulation using a kinetic model for batch cultivation and improving system performance using semi-batch cultivation. Bioprocess Biosyst Eng 45, 333–344 (2022). https://doi.org/10.1007/s00449-021-02663-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-021-02663-y