Abstract
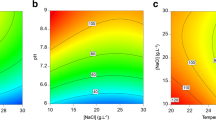
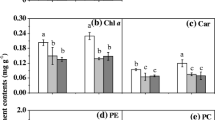
Chlamydomonas reinhardtii produces a variety of compounds that can be beneficial to human and animal health. Among these compounds, application of photosynthetic pigments, such as chlorophylls and carotenoids, has gained considerable interest in numerous industries. A better understanding on the interactive effects of essential nutrients and light on microalgal physiology and pigment production would be beneficial in improving cultivation strategies. Therefore, this study evaluated biomass, carotenoid and chlorophyll yield and the following fluorescence parameters: quantum yield in PS II [Y(II)] and electron transport rate (ETR) using response surface methodology (RSM). The Fv/Fm, Y(NO) and Y(NPQ) were also monitored; however, no significant relationship was observed. From the investigation it was apparent that nitrogen and carbon; as well as the interactive effects of (nitrogen and carbon) and (carbon and light irradiance) were significant factors. The model predicted the optimum conditions for maximum carotenoids (8.15 ± 0.389 mg g−1) were 08.7 mol l−1 of nitrogen, 0.2 mol l−1 and 50 μmol photon m−2 s−1 of light irradiance. While maximum chlorophyll (33.6 ± 0.854 mg g−1) required a higher nitrogen (11.21 mol l−1). The photosynthetic parameters [Y(II), ETR] was correlated with the primary pigments and biomass production. Increased photosynthetic activity was associated with high carbon and light. The Y(II)and ETR of PSII under these conditions were 0.2 and ~ 14, respectively. This approach was accurate in developing the model, optimizing factors and analysing interaction effects. This study served to provide a better understanding on the interactions between factors influencing pigment biosynthesis and photosynthetic performance of Chlamydomonas reinhardtii.





Similar content being viewed by others
Availability of data and material
The datasets generated during and/or analysed during this study are available from the corresponding author on reasonable request.
References
Gomaa MA, Al-Haj L, Abed RM (2016) Metabolic engineering of Cyanobacteria and microalgae for enhanced production of biofuels and high-value products. J Appl Microbiol 121:919–931
Banerjee S, Ray A, Das D (2021) Optimization of Chlamydomonas reinhardtii cultivation with simultaneous CO2 sequestration and biofuels production in a biorefinery framework. Sci Total Environ 762:143080
Mussagy CU, Winterburn J, Santos-Ebinuma VC, Pereira JFB (2019) Production and extraction of carotenoids produced by microorganisms. Appl Microbiol Biotechnol 103:1095–1114
Novoveska L, Ross ME, Stanley MS, Pradelles R, Wasiolek V, Sassi JF (2019) Microalgal carotenoids: a review of production, current markets, regulations, and future direction. Mar Drugs 17:640
Patel AK, Singhania RR, Sim SJ, Dong CD (2021) Recent advancements in mixotrophic bioprocessing for production of high value microalgal products. Bioresour Technol 320:124421
Sarkar S, Manna MS, Bhowmick TK, Gayen K (2020) Extraction of chlorophylls and carotenoids from dry and wet biomass of isolated Chlorella thermophila: optimization of process parameters and modelling by artificial neural network. Process Biochem 96:58–72
Silva SC, Ferreira I, Dias MM, Barreiro MF (2020) Microalgae-derived pigments: a 10-year bibliometric review and industry and market trend analysis. Molecules (Basel, Switzerland) 25:3406
Eismann AI, Perpetuo Reis R, Ferreira da Silva A, Negrão Cavalcanti D (2020) Ulva spp. carotenoids: responses to environmental conditions. Algal Res 48:101916
Cuellar-Bermudez SP, Aguilar-Hernandez I, Cardenas-Chavez DL, Ornelas-Soto N, Romero-Ogawa MA, Parra-Saldivar R (2015) Extraction and purification of high-value metabolites from microalgae: essential lipids, astaxanthin and phycobiliproteins. Microb Biotechnol 8:190–209
Nabi F, Arain MA, Rajput N, Alagawany M, Soomro J, Umer M, Soomro F, Wang Z, Ye R, Liu J (2020) Health benefits of carotenoids and potential application in poultry industry: a review. J Anim Physiol Anim Nutr 104:1809–1818
Couso I, Vila M, Vigara J, Cordero BF, Vargas MÁ, Rodríguez H, León R (2012) Synthesis of carotenoids and regulation of the carotenoid biosynthesis pathway in response to high light stress in the unicellular microalga Chlamydomonas reinhardtii. Eur J Phycol 47:223–232
Esquivel MG, Matos AR, Marques Silva J (2017) Rubisco mutants of Chlamydomonas reinhardtii display divergent photosynthetic parameters and lipid allocation. Appl Microbiol Biotechnol 101:5569–5580
Hang LT, Mori K, Tanaka Y, Morikawa M, Toyama T (2020) Enhanced lipid productivity of Chlamydomonas reinhardtii with combination of NaCl and CaCl2 stresses. Bioprocess Biosyst Eng 43:971–980
Kim EJ, Jung W, Lim S, Kim S, Han SJ, Choi H-G (2016) Growth and lipid content at low temperature of Arctic alga Chlamydomonas sp. KNM0029C. Bioprocess Biosyst Eng 39:151–157
Sigamani S, Natarajan H (2016) Bioactive compounds from microalgae and its different applications—a review. Adv Appl Sci Res 7:153–158
El-Mekkawi SA, Hussein HS, El-Enin SAA, El-Ibiari NN (2019) Assessment of stress conditions for carotenoids accumulation in Chlamydomonas reinhardtii as added-value algal products. Bulletin of the National Research Centre 43
Sun H, Mao X, Wu T, Ren Y, Chen F, Liu B (2018) Novel insight of carotenoid and lipid biosynthesis and their roles in storage carbon metabolism in Chlamydomonas reinhardtii. Bioresour Technol 263:450–457
Guarin-Villegas E, Remolina-Páez LM, Bermúdez-Castro JP, Mogollón-Londoño SO, Contreras-Ropero JE, García-Martínez JB, Barajas-Solano AF (2020) Effect of de carbon/nitrogen ratio on the production of microalgae-based carotenoids. Ingeniería y competitividad 22:12
Li X, Slavens S, Crunkleton DW, Johannes TW (2021) Interactive effect of light quality and temperature on Chlamydomonas reinhardtii growth kinetics and lipid synthesis. Algal Res 53:102127
Moon M, Kim CW, Park W-K, Yoo G, Choi Y-E, Yang J-W (2013) Mixotrophic growth with acetate or volatile fatty acids maximizes growth and lipid production in Chlamydomonas reinhardtii. Algal Res 2:352–357
Mandal S, Shurin JB, Efroymson RA, Mathews TJ (2018) Functional divergence in nitrogen uptake rates explains diversity–productivity relationship in microalgal communities. Ecosphere 9:e02228
Taghavi N, Robinson G (2016) Improving the optimum yield and growth of Chlamydomonas reinhardtii CC125 and CW15 using various carbon sources and growth regimes. Afr J Biotechnol 15:1083–1100
Mojaat M, Pruvost J, Foucault A, Legrand J (2008) Effect of organic carbon sources and Fe2+ ions on growth and β-carotene accumulation by Dunaliella salina. Biochem Eng J 39:177–184
Abd Wahab N, Mohd Ikhsan NF, Nagao N, Yusoff F, Shariff M, Banerjee S, Katayama T, Nakakuni M, Koyama M, Nakasaki K, Toda T (2021) Tolerance of tetraselmis tetrathele to high ammonium nitrogen and its effect on growth rate, carotenoid, and fatty acids productivity. Front Bioeng Biotechnol 9:19
Tevatia R, Demirel Y, Blum P (2012) Kinetic modeling of photoautotropic growth and neutral lipid accumulation in terms of ammonium concentration in Chlamydomonas reinhardtii. Bioresour Technol 119:419–424
Ramanna L, Rawat I, Zerrouki D, Bux F (2018) A novel organic dye-based approach to increase photon flux density for enhanced microalgal pigment production. J Clean Prod 198:187–194
Therien JB, Zadvornyy OA, Posewitz MC, Bryant DA, Peters JW (2014) Growth of Chlamydomonas reinhardtii in acetate-free medium when co-cultured with alginate-encapsulated, acetate-producing strains of Synechococcus sp. PCC 7002. Biotechnol Biofuels 7:154
Kong W, Yang S, Wang H, Huo H, Guo B, Liu N, Zhang A, Niu S (2020) Regulation of biomass, pigments, and lipid production by Chlorella vulgaris 31 through controlling trophic modes and carbon sources. J Appl Phycol 32:1569–1579
Raeisossadati M, Moheimani NR, Parlevliet D (2019) Red and blue luminescent solar concentrators for increasing Arthrospira platensis biomass and phycocyanin productivity in outdoor raceway ponds. Bioresour Technol 291:121801
Patel A, Matsakas L, Rova U, Christakopoulos P (2019) A perspective on biotechnological applications of thermophilic microalgae and cyanobacteria. Bioresour Technol 278:424–434
Anderson MJ, Whitcomb PJ (2017) DOE simplified: practical tools for effective experimentation. CRC Press, Boca Raton
Myers RH, Montgomery DC, Anderson-Cook CM (2016) Response surface methodology: process and product optimization using designed experiments. Wiley, New York
Yarnold J, Ross IL, Hankamer B (2016) Photoacclimation and productivity of Chlamydomonas reinhardtii grown in fluctuating light regimes which simulate outdoor algal culture conditions. Algal Res 13:182–194
Rathod JP, Vira C, Lali AM, Prakash G (2020) Metabolic engineering of Chlamydomonas reinhardtii for enhanced beta-carotene and lutein production. Appl Biochem Biotechnol 190:1457–1469
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592
Klughammer C, Schreiber U (2008) Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the saturation pulse method. PAM Appl Notes 1:27–35
Schreiber U, Klughammer C, Kolbowski J (2012) Assessment of wavelength-dependent parameters of photosynthetic electron transport with a new type of multi-color PAM chlorophyll fluorometer. Photosynth Res 113:127–144
Schuurmans RM, van Alphen P, Schuurmans JM, Matthijs HC, Hellingwerf KJ (2015) Comparison of the photosynthetic yield of cyanobacteria and green algae: different methods give different answers. PLoS ONE 10:e0139061
Behl K, SeshaCharan P, Joshi M, Sharma M, Mathur A, Kareya MS, Jutur PP, Bhatnagar A, Nigam S (2020) Multifaceted applications of isolated microalgae Chlamydomonas sp. TRC-1 in wastewater remediation, lipid production and bioelectricity generation. Bioresour Technol 304:122993
Ralph PJ, Gademann R (2005) Rapid light curves: a powerful tool to assess photosynthetic activity. Aquat Bot 82:222–237
Ratnapuram HP, Vutukuru SS, Yadavalli R (2018) Mixotrophic transition induced lipid productivity in Chlorella pyrenoidosa under stress conditions for biodiesel production. Heliyon 4:e00496
Pirastru L, Darwish M, Chu FL, Perreault F, Sirois L, Sleno L, Popovic R (2011) Carotenoid production and change of photosynthetic functions in Scenedesmus sp. exposed to nitrogen limitation and acetate treatment. J Appl Phycol 24:117–124
Chavoshi Z, Shariati M (2019) Lipid production in Dunaliella bardawil under autotrophic, heterotrophic and mixotrophic conditions. Braz J Oceanogr 67
Pang N, Gu X, Chen S, Kirchhoff H, Lei H, Roje S (2019) Exploiting mixotrophy for improving productivities of biomass and co-products of microalgae. Renew Sustain Energy Rev 112:450–460
Cecchin M, Benfatto S, Griggio F, Mori A, Cazzaniga S, Vitulo N, Delledonne M, Ballottari M (2018) Molecular basis of autotrophic vs mixotrophic growth in Chlorella sorokiniana. Sci Rep 8:6465
Cuaresma M, Casal C, Forján E, Vílchez C (2011) Productivity and selective accumulation of carotenoids of the novel extremophile microalga Chlamydomonas acidophila grown with different carbon sources in batch systems. J Ind Microbiol Biotechnol 38:167–177
García-Cañedo JC, Cristiani-Urbina E, Flores-Ortiz CM, Ponce-Noyola T, Esparza-García F, Cañizares-Villanueva RO (2016) Batch and fed-batch culture of Scenedesmus incrassatulus: effect over biomass, carotenoid profile and concentration, photosynthetic efficiency and non-photochemical quenching. Algal Res 13:41–52
Zhou Y, Schideman LC, Park DS, Stirbet A, Govindjee RSI, Krehbiel JD, Seufferheld MJ (2015) Characterization of a Chlamydomonas reinhardtii mutant strain with improved biomass production under low light and mixotrophic conditions. Algal Res 11:134–147
Roach T, Na CS, Stoggl W, Krieger-Liszkay A (2020) The non-photochemical quenching protein LHCSR3 prevents oxygen-dependent photoinhibition in Chlamydomonas reinhardtii. J Exp Bot 71:2650–2660
Heifetz PB, Förster B, Osmond CB, Giles LJ, Boynton JE (2000) Effects of acetate on facultative autotrophy in Chlamydomonas reinhardtii assessed by photosynthetic measurements and stable isotope analyses. Plant Physiol 122:1439–1445
Li M, Wilkins MR (2020) Recent advances in polyhydroxyalkanoate production: feedstocks, strains and process developments. Int J Biol Macromol 156:691–703
Li P, Sun X, Sun X, Tang J, Turaib A, Wang X, Cheng Z, Deng L, Zhang Y (2020) Response of lipid productivity to photosynthesis of Chlorella vulgaris under various nutrient stress modes. J Renew Sustain Energy 12:056102
Wang Q, Sonobe R (2016) Tracing photosynthetic electron transport rate based on hyperspectral reflectance. In: 2016 IEEE international geoscience and remote sensing symposium (IGARSS), pp 1723–1726
Li T, Kirchhoff H, Gargouri M, Feng J, Cousins AB, Pienkos PT, Gang DR, Chen S (2016) Assessment of photosynthesis regulation in mixotrophically cultured microalga Chlorella sorokiniana. Algal Res 19:30–38
Kruskopf M, Flynn KJ (2006) Chlorophyll content and fluorescence responses cannot be used to gauge reliably phytoplankton biomass, nutrient status or growth rate. New Phytol 169:525–536
Lee DY, Park J-J, Barupal DK, Fiehn O (2012) System response of metabolic networks in Chlamydomonas reinhardtii to total available ammonium. Mol Cell Proteomics 11:973–988
Yaakob MA, Mohamed R, Al-Gheethi A, Aswathnarayana Gokare R, Ambati RR (2021) Influence of nitrogen and phosphorus on microalgal growth, biomass, lipid, and fatty acid production: an overview. Cells 10:393
Mulders KJM, Janssen JH, Martens DE, Wijffels RH, Lamers PP (2014) Effect of biomass concentration on secondary carotenoids and triacylglycerol (TAG) accumulation in nitrogen-depleted Chlorella zofingiensis. Algal Res 6:8–16
Brzezowski P, Richter AS, Grimm B (2015) Regulation and function of tetrapyrrole biosynthesis in plants and algae. Biochim Biophys Acta (BBA) Bioenerg 1847:968–985
Kim S-H, Liu K-H, Lee S-Y, Hong S-J, Cho B-K, Lee H, Lee C-G, Choi H-K (2013) Effects of light intensity and nitrogen starvation on glycerolipid, glycerophospholipid, and carotenoid composition in Dunaliella tertiolecta culture. PLoS ONE 8:e72415
Zhu S, Huang W, Xu J, Wang Z, Xu J, Yuan Z (2014) Metabolic changes of starch and lipid triggered by nitrogen starvation in the microalga Chlorella zofingiensis. Bioresour Technol 152:292–298
Kamalanathan M, Pierangelini M, Shearman LA, Gleadow R, Beardall J (2016) Impacts of nitrogen and phosphorus starvation on the physiology of Chlamydomonas reinhardtii. J Appl Phycol 28:1509–1520
Ferreira VS, Pinto RF, Sant’Anna C (2016) Low light intensity and nitrogen starvation modulate the chlorophyll content of Scenedesmus dimorphus. J Appl Microbiol 120:661–670
Msanne J, Xu D, Konda AR, Casas-Mollano JA, Awada T, Cahoon EB, Cerutti H (2012) Metabolic and gene expression changes triggered by nitrogen deprivation in the photoautotrophically grown microalgae Chlamydomonas reinhardtii and Coccomyxa sp. C-169. Phytochemistry 75:50–59
Tanaka A, Tanaka R (2006) Chlorophyll metabolism. Curr Opin Plant Biol 9:248–255
Hörtensteiner S (1999) Chlorophyll breakdown in higher plants and algae. Cell Mol Life Sci CMLS 56:330–347
Acknowledgements
The authors thank our colleagues at the Institute for Water and Wastewater Technology (IWWT) and Durban University of Technology (DUT) for their support and guidance.
Funding
This study was funded by the National Research Foundation (NRF-SARChi), Grant Number 84166.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by TM, VB and LR. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests or competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mogany, T., Bhola, V., Ramanna, L. et al. Photosynthesis and pigment production: elucidation of the interactive effects of nutrients and light on Chlamydomonas reinhardtii. Bioprocess Biosyst Eng 45, 187–201 (2022). https://doi.org/10.1007/s00449-021-02651-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-021-02651-2