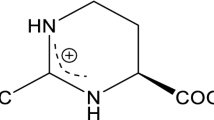
Abstract
In this study, the recombinant ectoine-producing Escherichia coli ET01 was constructed by introducing the ectABC operon from Halomonas venusta ZH. To further improve ectoine production, the regulation of the fermentation process was systematically investigated. First, the effects of the initial glucose concentrations and glucose feeding mode on ectoine production were analyzed. Using a combination of pH-feedback feeding and glucose-controlled feeding, the ectoine titer reached 25.5 g/L, representing an 8.8-fold increase over standard batch culture. Then, the effects of dissolved oxygen (DO) levels (50, 40, 30, or 20%) on ectoine production were studied, and a DO control strategy was developed based on the fermentation kinetics. When the final optimized two-stage fermentation strategy was used, the ectoine titer reached 47.8 g/L, which was the highest level of ectoine produced by E. coli fermentation. The fermentation regulation strategy developed in this study might be useful for scaling up the commercial production of ectoine.







Similar content being viewed by others
References
Held C, Sadowski G (2016) Compatible solutes: thermodynamic properties relevant for effective protection against osmotic stress. Fluid Phase Equilib 407:224–235
Oren A (2011) Thermodynamic limits to microbial life at high salt concentrations. Environ Microbiol 13:1908–1923
Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN (1982) Living with water stress: evolution of osmolyte systems. Science 217:1214–1222
Yancey PH, Gerringer ME, Drazen JC, Rowden AA, Jamieson A (2014) Marine fish may be biochemically constrained from inhabiting the deepest ocean depths. Proc Natl Acad Sci 111:4461–4465
Czech L, Hermann L, Stöveken N, Richter A, Höppner A, Smits S, Heider J, Bremer E (2018) Role of the extremolytes ectoine and hydroxyectoine as stress protectants and nutrients: genetics, phylogenomics, biochemistry, and structural analysis. Genes 9:177
Da Costa MS, Santos H, Galinski EA (1998). An overview of the role and diversity of compatible solutes in Bacteria and Archaea. In Biotechnology of extremophiles. (Springer), pp. 117–153.
Kempf B, Bremer, E.J.A.o.m. (1998) Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch Microbiol 170:319–330
Mais CN, Hermann L, Altegoer F, Seubert A, Richter AA, Wernersbach I, Czech L, Bremer E, Bange G (2020) Degradation of the microbial stress protectants and chemical chaperones ectoine and hydroxyectoine by a bacterial hydrolase-deacetylase complex. J Biol Chem 295:9087–9104
Widderich N, Hoeppner A, Pittelkow M, Heider J, Smits SH, Bremer E (2014) Biochemical properties of ectoine hydroxylases from extremophiles and their wider taxonomic distribution among microorganisms. PloS One 9:e93809
Boroujeni MB, Nayeri H (2018) Stabilization of bovine lactoperoxidase in the presence of ectoine. Food Chem 265:208–215
Graf R, Anzali S, Buenger J, Pfluecker F, Driller H (2008) The multifunctional role of ectoine as a natural cell protectant. Clin Dermatol 26:326–333
Brands S, Schein P, Castro-Ochoa KF, Galinski EA (2019) Hydroxyl radical scavenging of the compatible solute ectoine generates two N-acetimides. Arch Biochem Biophys 674:108097
Unfried K, Krämer U, Sydlik U, Autengruber A, Bilstein A, Stolz S, Marini A, Schikowski T, Keymel S, Krutmann J (2016) Reduction of neutrophilic lung inflammation by inhalation of the compatible solute ectoine: a randomized trial with elderly individuals. Int J Chron Obstruct Pulmon Dis 11:2573
Wedeking A, Hagen-Euteneuer N, Gurgui M, Broere R, Lentzen G, Tolba R, Galinski E, van Echten-Deckert G (2014) A lipid anchor improves the protective effect of ectoine in inflammation. Curr Med Chem 21:2565–2572
Bownik A, Stępniewska Z (2016) Ectoine as a promising protective agent in humans and animals. Arch Ind Hyg Toxicol 67:260–265
Marini A, Reinelt K, Krutmann J, Bilstein A (2014) Ectoine-containing cream in the treatment of mild to moderate atopic dermatitis: a randomised, comparator-controlled, intra-individual double-blind, multi-center trial. Skin Pharmacol Physiol 27:57–65
Becker J, Wittmann, C.J.C.O.i.B. (2020) Microbial production of extremolytes—high-value active ingredients for nutrition, health care, and well-being. Curr Opin Biotechnol 65:118–128
Pastor JM, Salvador M, Argandoña M, Bernal V, Reina-Bueno M, Csonka LN, Iborra JL, Vargas C, Nieto JJ, Cánovas, M.J.B.a. (2010) Ectoines in cell stress protection: uses and biotechnological production. Biotechnol Adv 28:782–801
Hermann L, Mais C-N, Czech L, Smits SH, Bange G, Bremer EJBC (2020) The ups and downs of ectoine: structural enzymology of a major microbial stress protectant and versatile nutrient. Biol Chem 401:1443–1468
Peters P, Galinski E, Trüper HJ (1990) The biosynthesis of ectoine. FEMS Microbiol Lett 71:157–162
Richter AA, Kobus S, Czech L, Hoeppner A, Zarzycki J, Erb TJ, Lauterbach L, Dickschat JS, Bremer E, Smits SH (2020) The architecture of the diaminobutyrate acetyltransferase active site provides mechanistic insight into the biosynthesis of the chemical chaperone ectoine. J Biol Chem 295:2822–2838
Sauer T, Galinski EAJB (1998) Bacterial milking: a novel bioprocess for production of compatible solutes. Biotechnol Bioeng 57:306–313
Grammann K, Volke A, Kunte HJJJ (2002) New type of osmoregulated solute transporter identified in halophilic members of the bacteria domain: TRAP transporter TeaABC mediates uptake of ectoine and hydroxyectoine in Halomonas elongata DSM 2581T. J Bacteriol 184:3078–3085
Kunte HJ, Lentzen G, Galinski E (2014) Industrial production of the cell protectant ectoine: protection mechanisms, processes, and products. Curr Biotechnol 3:10–15
Fischel U, Oren A (1993) Fate of compatible solutes during dilution stress in Ectothiorhodospira marismortui. FEMS Microbiol Lett 113:113–118
Becker J, Schäfer R, Kohlstedt M, Harder BJ, Borchert NS, Stöveken N, Bremer E, Wittmann CJ (2013) Systems metabolic engineering of Corynebacterium glutamicum for production of the chemical chaperone ectoine. Microb Cell Fact 12:110
Czech L, Poehl S, Hub P, Stoeveken N, Bremer EJA (2018) Tinkering with osmotically controlled transcription allows enhanced production and excretion of ectoine and hydroxyectoine from a microbial cell factory. Appl Environ Microbiol 84:e01772
Eilert E, Kranz A, Hollenberg CP, Piontek M, Suckow MJ (2013) Synthesis and release of the bacterial compatible solute 5-hydroxyectoine in Hansenula polymorpha. J Biotechnol 167:85–93
Giesselmann G, Dietrich D, Jungmann L, Kohlstedt M, Jeon EJ, Yim SS, Sommer F, Zimmer D, Muhlhaus T, Schroda M et al (2019) Metabolic engineering of Corynebacterium glutamicum for high-level ectoine production: design, combinatorial assembly, and implementation of a transcriptionally balanced heterologous ectoine pathway. Biotechnol J 14:e1800417
He J, Huang X, Gu L, Jiang J, Li SJ (2006) Cloning of the ectoine biosynthesis gene ectABC from Halomonas sp. BYS-1 and salt stressed expression in E. coli. Wei Sheng Wu Xue Bao 46:28
He Y-Z, Gong J, Yu H-Y, Tao Y, Zhang S, Dong Z-Y (2015) High production of ectoine from aspartate and glycerol by use of whole-cell biocatalysis in recombinant Escherichia coli. Microb Cell Fact 14:1–10
Ning Y, Wu X, Zhang C, Xu Q, Chen N, Xie X (2016) Pathway construction and metabolic engineering for fermentative production of ectoine in Escherichia coli. Metab Eng 36:10–18
Parwata IP, Wahyuningrum D, Suhandono S, Hertadi RJ (2019) Heterologous ectoine production in Escherichia coli: optimization using response surface methodology. Int J Microbiol 2019:5475361
Perez-Garcia F, Ziert C, Risse JM, Wendisch VF (2017) Improved fermentative production of the compatible solute ectoine by Corynebacterium glutamicum from glucose and alternative carbon sources. J Biotechnol 258:59–68
Schubert T, Maskow T, Benndorf D, Harms H, Breuer U (2007) Continuous synthesis and excretion of the compatible solute ectoine by a transgenic, nonhalophilic bacterium. Appl Environ Microbiol 73:3343–3347
Czech L, Stoveken N, Bremer E (2016) EctD-mediated biotransformation of the chemical chaperone ectoine into hydroxyectoine and its mechanosensitive channel-independent excretion. Microb Cell Fact 15:126
Czech L, Poehl S, Hub P, Stoveken N, Bremer E (2018) Tinkering with osmotically controlled transcription allows enhanced production and excretion of ectoine and hydroxyectoine from a microbial cell factory. Appl Environ Microbiol 84:e01772
Vandrich J, Pfeiffer F, Alfaro-Espinoza G, Kunte HJ (2020) Contribution of mechanosensitive channels to osmoadaptation and ectoine excretion in Halomonas elongata. Extremophiles 24:421–432
Jebbar M, Talibart R, Gloux K, Bernard T, Blanco CJ (1992) Osmoprotection of Escherichia coli by ectoine: uptake and accumulation characteristics. J Bacteriol 174:5027–5035
Tan R, Lyu Y, Zeng W, Zhou J (2019) Enhancing scleroglucan production by Sclerotium rolfsii WSH-G01 through a pH-shift strategy based on kinetic analysis. Biores Technol 293:122098
Zhang J, Liu L, Li J, Du G, Chen J (2012) Enhanced glucosamine production by Aspergillus sp. BCRC 31742 based on the time-variant kinetics analysis of dissolved oxygen level. Biores Technol 111:507–511
Zhu Y, Liu Y, Li J, Shin H-D, Du G, Liu L, Chen J (2015) An optimal glucose feeding strategy integrated with step-wise regulation of the dissolved oxygen level improves N-acetylglucosamine production in recombinant Bacillus subtilis. Biores Technol 177:387–392
Qiu Y, Zhang Y, Zhu Y, Sha Y, Xu H (2019) Improving poly-(γ-glutamic acid) production from a glutamic acid-independent strain from inulin substrate by consolidated bioprocessing. Bioprocess Biosyst Eng 42:1711–1720
Wang T, Lu Y, Yan H, Li X, Lü X (2019) Fermentation optimization and kinetic model for high cell density culture of a probiotic microorganism: Lactobacillus rhamnosus LS-8. Bioprocess Biosyst Eng 43:515–528
Amann E, Ochs B, Abel K-JJG (1988) Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene 69:301–315
Riesenberg D (1991) High-cell-density cultivation of Escherichia coli. Curr Opin Biotechnol 2:380–384
Ding S, Tan T (2006) L-lactic acid production by Lactobacillus casei fermentation using different fed-batch feeding strategies. Process Biochem 41:1451–1454
Korz D, Rinas U, Hellmuth K, Sanders E, Deckwer W-D (1995) Simple fed-batch technique for high cell density cultivation of Escherichia coli. J Biotechnol 39:59–65
Qu L, Ren L-J, Sun G-N, Ji X-J, Nie Z-K, Huang H (2013) Batch, fed-batch and repeated fed-batch fermentation processes of the marine thraustochytrid Schizochytrium sp. for producing docosahexaenoic acid. Bioprocess Biosyst Eng 36:1905–1912
Paalme T, Tiisma K, Kahru A, Vanatalu K, Vilu R (1990) Glucose-limited fed-batch cultivation of Escherichia coli with computer-controlled fixed growth rate. Biotechnol Bioeng 35:312–319
Shiloach J, Kaufman J, Guillard A, Fass R (1996) Effect of glucose supply strategy on acetate accumulation, growth, and recombinant protein production by Escherichia coli BL21 (λDE3) and Escherichia coli JM109. Biotechnol Bioeng 49:421–428
Chen X, Liu L, Li J, Du G, Chen J (2012) Improved glucosamine and N-acetylglucosamine production by an engineered Escherichia coli via step-wise regulation of dissolved oxygen level. Biores Technol 110:534–538
Eiteman MA, Altman E (2006) Overcoming acetate in Escherichia coli recombinant protein fermentations. Trends Biotechnol 24:530–536
Fallet C, Rohe P, Franco-Lara EJB (2010) Process optimization of the integrated synthesis and secretion of ectoine and hydroxyectoine under hyper/hypo-osmotic stress. Biotechnol Bioeng 107:124–133
Acknowledgements
This work was supported by the National Key R&D Program of China (No. 2019YFA0905203), the National Natural Science Foundation of China (No. 21776133), the Jiangsu Synergetic Innovation Center for Advanced Bio-Manufacture (No. XTB1804).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dong, Y., Zhang, H., Wang, X. et al. Enhancing ectoine production by recombinant Escherichia coli through step-wise fermentation optimization strategy based on kinetic analysis. Bioprocess Biosyst Eng 44, 1557–1566 (2021). https://doi.org/10.1007/s00449-021-02541-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-021-02541-7