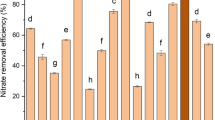
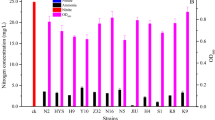
Abstract
A novel Pseudomonas sp. GZWN4 with the aerobic nitrogen removal ability was isolated from aquaculture water, whose removal efficiency of NO2−–N, NO3−–N and NH4+–N was 99.72%, 82.54% and 98.62%, respectively. The key genes involved in nitrogen removal, nxr, napA, narI, nirS, norB and nosZ, were successfully amplified and by combination with the results of nitrogen balance analysis, it was inferred that the denitrification pathway of strain GZWN4 was NO3−–N → NO2−–N → NO → N2O → N2. The strain GZWN4 had excellent nitrite removal performance at pH 7.0–8.5, temperature 25–30 ℃, C/N ratio 5–20, salinity 8–32‰ and dissolved oxygen concentration 2.52–5.73 mg L−1. The receivable linear correlation (R2 = 0.9809) was obtained with the range of quantification between l03 and 108 CFU mL−1 of the strain by enzyme-linked immunosorbent assay. Strain GZWN4 could maintain high abundance in the actual water and wastewater of mariculture and the removal efficiency of TN were 52.57% and 63.64%, respectively. The safety evaluation experiment showed that the strain GZWN4 had no hemolysis and high biosecurity toward shrimp Litopenaeus vannamei. The excellent nitrogen removal ability and adaptability to aquaculture environment made strain GZWN4 a promising candidate for treatment of water and wastewater in aquaculture.








Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Nations FAO (2019) Fishery and aquaculture statistics 2017. Food & Agriculture Organization of the United Nations
Martínez-Córdova LR, Emerenciano M, Miranda-Baeza A, Martínez-Porchas M (2015) Microbial-based systems for aquaculture of fish and shrimp: an updated review. Rev Aquac 7(2):131–148. https://doi.org/10.1111/raq.12058
Lin Y-C, Chen J-C (2003) Acute toxicity of nitrite on Litopenaeus vannamei (Boone) juveniles at different salinity levels. Aquaculture 224(1–4):193–201
Kuypers MMM, Marchant HK, Kartal B (2018) The microbial nitrogen-cycling network. Nat Rev Microbiol 16(5):263–276. https://doi.org/10.1038/nrmicro.2018.9
Ciji A, Akhtar MS (2019) Nitrite implications and its management strategies in aquaculture: a review. Rev Aquac 12(2):878–908. https://doi.org/10.1111/raq.12354
Peng Y, Zhu G (2006) Biological nitrogen removal with nitrification and denitrification via nitrite pathway. Appl Microbiol Biotechnol 73(1):15–26. https://doi.org/10.1007/s00253-006-0534-z
Khin T, Annachhatre AP (2004) Novel microbial nitrogen removal processes. Biotechnol Adv 22(7):519–532. https://doi.org/10.1016/j.biotechadv.2004.04.003
Yang X, Wang S, Zhou L (2012) Effect of carbon source, C/N ratio, nitrate and dissolved oxygen concentration on nitrite and ammonium production from denitrification process by Pseudomonas stutzeri D6. Bioresour Technol 104:65–72. https://doi.org/10.1016/j.biortech.2011.10.026
Chen J, Gu S, Hao H, Chen J (2016) Characteristics and metabolic pathway of Alcaligenes sp. TB for simultaneous heterotrophic nitrification-aerobic denitrification. Appl Microbiol Biotechnol 100(22):9787–9794. https://doi.org/10.1007/s00253-016-7840-x
Huang F, Pan L, Lv N, Tang X (2017) Characterization of novel Bacillus strain N31 from mariculture water capable of halophilic heterotrophic nitrification–aerobic denitrification. J Biosci Bioeng 124(5):564–571. https://doi.org/10.1016/j.jbiosc.2017.06.008
Zhang M, Li A, Yao Q, Wu Q, Zhu H (2020) Nitrogen removal characteristics of a versatile heterotrophic nitrifying-aerobic denitrifying bacterium, Pseudomonas bauzanensis DN13-1, isolated from deep-sea sediment. Biores Technol. https://doi.org/10.1016/j.biortech.2019.122626
Qi Z, Zhang X-H, Boon N, Bossier P (2009) Probiotics in aquaculture of China—current state, problems and prospect. Aquaculture 290(1–2):15–21. https://doi.org/10.1016/j.aquaculture.2009.02.012
Tal Y, Schwartsburd B, Nussinovitch A (2001) Enumeration and factors influencing the relative abundance of a denitrifier, Pseudomonas sp. JR12, entrapped in alginate beads. Environ Pollut 112(2):99–106
Skjermo J, Bakke I, Dahle SW, Vadstein O (2015) Probiotic strains introduced through live feed and rearing water have low colonizing success in developing Atlantic cod larvae. Aquaculture 438:17–23. https://doi.org/10.1016/j.aquaculture.2014.12.027
Kim S-A, Bae J-H, Seong H, Han NS (2020) Development of Leuconostoc lactis–specific quantitative PCR and its application for identification and enumeration in fermented foods. Food Anal Methods 13(4):992–999. https://doi.org/10.1007/s12161-020-01720-8
Silvafroufe LGD, Boddey RM, Reis VM (2009) Quantification of natural populations of Gluconacetobacter diazotrophicus and Herbaspirillum spp. In sugar cane (Saccharum spp.) Using differente polyclonal antibodies. Braz J Microbiol 40(4):866–878
Su Z, Li Y, Pan L, Xue F (2016) An investigation on the immunoassays of an ammonia nitrogen-degrading bacterial strain in aquatic water. Aquaculture 450:17–22. https://doi.org/10.1016/j.aquaculture.2015.07.001
Stanley J, Baquar N, Burnens A (1995) Molecular subtyping scheme for Salmonella panama. J Clin Microbiol 33(5):1206–1211
Dong X, Cai M (2001) Determinative manual for routine bacteriology. Scientific Press, Beijing (English translation)
Manoj V (2013) Detection of Denitrifying population from upflow anaerobic packed bed column using PCR. Int J Curr Microbiol Appl Sci 2(11):206–217
Liu X, Lianxing LI, Xianglan BO, Chen J, Zhou S, Wang J, Wang L, Chen C (2018) Functional genes of heterotrophic nitrification and aerobic denitrification in bacterium Pseudomon asaeruginosa. Fish Sci 37(4):475–483
Rice BE, Rollins DM, Edward T (1997) Campylobacter jejuni in broiler chickens: colonization and humoral immunity following oral vaccination and experimental infection. Vaccine 15(17–18):1922
Yong-Qin LI, Sheng XZ, Zhan WB, Xiao-Li XU (2010) Construction of immunochip for detection of six fish bacterial pathogens. Period Ocean Univ China 40(8):48–54
Xu W-J, Pan L-Q (2014) Evaluation of dietary protein level on selected parameters of immune and antioxidant systems, and growth performance of juvenile Litopenaeus vannamei reared in zero-water exchange biofloc-based culture tanks. Aquaculture 426–427:181–188. https://doi.org/10.1016/j.aquaculture.2014.02.003
APHA (1998) Standard methods for the examination of water and wastewater. 20^ Edition, Washington, Dc
Rout PR, Bhunia P, Dash RR (2017) Simultaneous removal of nitrogen and phosphorous from domestic wastewater using Bacillus cereus GS-5 strain exhibiting heterotrophic nitrification, aerobic denitrification and denitrifying phosphorous removal. Bioresour Technol. https://doi.org/10.1016/j.biortech.2017.07.186
Huang T, Guo L, Zhang H, Su J, Wen G, Zhang K (2015) Nitrogen-removal efficiency of a novel aerobic denitrifying bacterium, Pseudomonas stutzeri strain ZF31, isolated from a drinking-water reservoir. Bioresour Technol 196:209–216. https://doi.org/10.1016/j.biortech.2015.07.059
Jia Y, Zhou M, Chen Y, Luo J, Hu Y (2019) Carbon selection for nitrogen degradation pathway by Stenotrophomonas maltophilia: Based on the balances of nitrogen, carbon and electron. Bioresour Technol 294:122114. https://doi.org/10.1016/j.biortech.2019.122114
Tsukuda S, Christianson L, Kolb A, Saito K, Summerfelt S (2015) Heterotrophic denitrification of aquaculture effluent using fluidized sand biofilters. Aquac Eng 64:49–59. https://doi.org/10.1016/j.aquaeng.2014.10.010
Song ZF, An J, Fu GH, Yang XL (2011) Isolation and characterization of an aerobic denitrifying Bacillus sp. YX-6 from shrimp culture ponds. Aquaculture 319(1–2):188–193. https://doi.org/10.1016/j.aquaculture.2011.06.018
Zheng HY, Liu Y, Gao XY, Ai GM, Miao LL, Liu ZP (2012) Characterization of a marine origin aerobic nitrifying-denitrifying bacterium. J Biosci Bioeng 114(1):33–37. https://doi.org/10.1016/j.jbiosc.2012.02.025
Zhao B, Cheng DY, Tan P, An Q, Guo JS (2018) Characterization of an aerobic denitrifier Pseudomonas stutzeri strain XL-2 to achieve efficient nitrate removal. Bioresour Technol 250:564–573. https://doi.org/10.1016/j.biortech.2017.11.038
Wan C, Yang X, Lee DJ, Du M, Wan F, Chen C (2011) Aerobic denitrification by novel isolated strain using NO2–N as nitrogen source. Bioresour Technol 102(15):7244–7248. https://doi.org/10.1016/j.biortech.2011.04.101
Bai H, Liao S, Wang A, Huang J, Shu W, Ye J (2019) High-efficiency inorganic nitrogen removal by newly isolated Pannonibacter phragmitetus B1. Bioresour Technol 271:91–99. https://doi.org/10.1016/j.biortech.2018.09.090
Su JJ, Yeh KS, Tseng PW (2006) A strain of Pseudomonas sp. isolated from piggery wastewater treatment systems with heterotrophic nitrification capability in Taiwan. Curr Microbiol 53(1):77–81. https://doi.org/10.1007/s00284-006-0021-x
Koo J-G, Kim S-G, Jee J-H, Kim J-M, Bai SC, Kang J-C (2005) Effects of ammonia and nitrite on survival, growth and moulting in juvenile tiger crab, Orithyia sinica (Linnaeus). Aquac Res 36(1):79–85. https://doi.org/10.1111/j.1365-2109.2004.01187.x
Zou S, Yao S, Ni J (2014) High-efficient nitrogen removal by coupling enriched autotrophic-nitrification and aerobic-denitrification consortiums at cold temperature. Bioresour Technol 161:288–296. https://doi.org/10.1016/j.biortech.2014.03.066
Soto O, Aspé E, Roeckel M (2007) Kinetics of cross-inhibited denitrification of a high load wastewater. Enzyme Microb Technol 40(6):1627–1634. https://doi.org/10.1016/j.enzmictec.2006.11.014
Ruan Y, Taherzadeh MJ, Kong D, Lu H, Zhao H, Xu X, Liu Y, Cai L (2020) Nitrogen removal performance and metabolic pathways analysis of a novel aerobic denitrifying halotolerant Pseudomonas balearica strain RAD-17. Microorganisms. https://doi.org/10.3390/microorganisms8010072
Xia L, Li X, Fan W, Wang J (2020) Heterotrophic nitrification and aerobic denitrification by a novel Acinetobacter sp. ND7 isolated from municipal activated sludge. Bioresour Technol 301:122749. https://doi.org/10.1016/j.biortech.2020.122749
Ji B, Yang K, Zhu L, Jiang Y, Wang H, Zhou J, Zhang H (2015) Aerobic denitrification: a review of important advances of the last 30 years. Biotechnol Bioprocess Eng 20(4):643–651. https://doi.org/10.1007/s12257-015-0009-0
Fang FC (2004) Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol 2(10):820–832
He T, Xie D, Li Z, Ni J, Sun Q (2017) Ammonium stimulates nitrate reduction during simultaneous nitrification and denitrification process by Arthrobacter arilaitensis Y-10. Bioresour Technol 239:66–73. https://doi.org/10.1016/j.biortech.2017.04.125
Ye J, Zhao B, An Q, Huang YS (2016) Nitrogen removal by Providencia rettgeri strain YL with heterotrophic nitrification and aerobic denitrification. Environ Technol 37(17):2206–2213. https://doi.org/10.1080/09593330.2016.1146338
Kim M, Jeong SY, Yoon SJ, Cho SJ, Kim YH, Kim MJ, Ryu EY, Lee SJ (2008) Aerobic denitrification of Pseudomonas putida AD-21 at different C/N ratios. J Biosci Bioeng 106(5):498–502. https://doi.org/10.1263/jbb.106.498
Kaya D, Genc MA, Aktas M, Yavuzcan H, Ozmen O, Genc E (2019) Effect of biofloc technology on growth of speckled shrimp, Metapenaeus monoceros (Fabricus) in different feeding regimes. Aquac Res 50(10):2760–2768. https://doi.org/10.1111/are.14228
Gui M, Chen Q, Ni J (2017) Effect of NaCl on aerobic denitrification by strain Achromobacter sp. GAD-3. Appl Microbiol Biotechnol 101(12):5139–5147. https://doi.org/10.1007/s00253-017-8191-y
Ka JO, Urbance J, Ye RW et al (1997) Diversity of oxygen and n-oxide regulation of nitrite reductases in denitrifying bacteria. FEMS Microbiol Lett. https://doi.org/10.1111/j.1574-6968.1997.tb12705
Sun Y, Li A, Zhang X, Ma F (2015) Regulation of dissolved oxygen from accumulated nitrite during the heterotrophic nitrification and aerobic denitrification of pseudomonas stutzeri t13. Appl Microbiol Biotechnol 99(7):3243–3248. https://doi.org/10.1007/s00253-014-6221-6
Rkü I, Timur G (2014) A comparative study on detection methods of Lactococcus garvieae in experimentally infected rainbow trout (Oncorhynchus mykiss, W.). Bamidgeh 66:IJA_66.2014.1025 2010 pages
Shyne Anand PS, Sobhana KS, George KC, Paul Raj R (2016) Selection of specific cell wall antigen for rapid detection of fish pathogenic Vibrio parahaemolyticus by enzyme immunoassay. Indian J Fish. https://doi.org/10.21077/ijf.2016.63.2.44334-11
Hochel I, Viochna D, Škvor J, Musil M (2004) Development of an indirect competitive ELISA for detection of Campylobacter jejuni sub sp. O:23 in foods. Folia Microbiol 49(5):579–586
Rout PR, Dash RR, Bhunia P, Rao S (2018) Role of Bacillus cereus GS-5 strain on simultaneous nitrogen and phosphorous removal from domestic wastewater in an inventive single unit multi-layer packed bed bioreactor. Bioresour Technol 262:251–260. https://doi.org/10.1016/j.biortech.2018.04.087
Huang H, Lin Q, Guo Z, Zhuojia LI, Xiaoyu LU, Yang M, Jia X (2007) Effects of probiotics on the dynamic of bacteria in marine shrimp pond. South China Fish Sci 3(3):14–19
Courvalin P (2006) Antibiotic resistance: the pros and cons of probiotics. Dig Liver Dis 38:S261–S265. https://doi.org/10.1016/s1590-8658(07)60006-1
Yun L, Yu Z, Li Y, Luo P, Jiang X, Tian Y, Ding X (2019) Ammonia nitrogen and nitrite removal by a heterotrophic Sphingomonas sp. strain LPN080 and its potential application in aquaculture. Aquaculture 500:477–484. https://doi.org/10.1016/j.aquaculture.2018.10.054
Mogrovejo DC, Perini L, Gostincar C, Sepcic K, Turk M, Ambrozic-Avgustin J, Brill FHH, Gunde-Cimerman N (2020) Prevalence of antimicrobial resistance and hemolytic phenotypes in culturable arctic bacteria. Front Microbiol 11:570. https://doi.org/10.3389/fmicb.2020.00570
Fernandes S, Kerkar S, Leitao J, Mishra A (2019) Probiotic role of salt pan bacteria in enhancing the growth of whiteleg shrimp, Litopenaeus vannamei. Probiotics Antimicrob Proteins 11(4):1309–1323. https://doi.org/10.1007/s12602-018-9503-y
Acknowledgements
This work was supported by project of Bio-Form biotechnology (Shandong) Co. LTD (20160359).
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. All the authors read and approved the final manuscript. ZS: conceptualization, methodology, investigation, formal analysis, writing—original draft and project administration; YL: supervision, conceptualization, and writing—review and editing; LP: investigation and formal analysis; ZH: investigation; LD: investigation; LL: investigation; MZ: investigation.
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest to declare.
Ethical approval
The experiment was permitted by the Institutional Animal Care and Use Committee of the Ocean University of China.
Consent for publication
All the authors agreed to publish.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Su, Z., Li, Y., Pan, L. et al. Nitrogen removal performance, quantitative detection and potential application of a novel aerobic denitrifying strain, Pseudomonas sp. GZWN4 isolated from aquaculture water. Bioprocess Biosyst Eng 44, 1237–1251 (2021). https://doi.org/10.1007/s00449-021-02523-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-021-02523-9