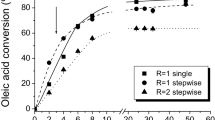
Abstract
The lipolytic yeast Yarrowia lipolytica produces cell-wall-associated lipases, namely Lip7p and Lip8p, that could have interesting properties as catalyst either in free (released lipase fraction—RLF) or cell-associated (cell-bound lipase fraction—CBLF) forms. Herein, a mixture of waste soybean frying oil, yeast extract and bactopeptone was found to favor the enzyme production. Best parameters for lipase activation and release from the cell wall by means of acoustic wave treatment were defined as: 26 W/cm2 for 1 min for CBLF and 52 W/cm2 for 2 min for RLF. Optimal pH and temperature values for lipase activity together with storage conditions were similar for both the free enzyme and cell-associated one: pH 7.0; T = 37 °C; and > 70% residual activity for 60 days at 4, − 4 °C and for 15 days at 30 °C.




Similar content being viewed by others
Data availability
All data are available for transparency.
References
Divakar S, Manohar B (2007) Use of lipases in the industrial production of esters. Ind Enzym Struct Funct Appl. https://doi.org/10.1007/1-4020-5377-0_17
Ollis DL, Cheah E, Cygler M et al (1992) The a/|3 hydrolase fold. Protein Eng 5:197–211
Arpigny JL, Jaeger KE (1999) Bacterial lipolytic enzymes: classification and properties. Biochem J 343:177–183. https://doi.org/10.1042/0264-6021:3430177
Fickers P, Marty A, Nicaud JM (2011) The lipases from Yarrowia lipolytica: genetics, production, regulation, biochemical characterization and biotechnological applications. Biotechnol Adv 29:632–644. https://doi.org/10.1016/j.biotechadv.2011.04.005
Ribeiro BD, De Castro AM, Coelho MAZ, Freire DMG (2011) Production and use of lipases in bioenergy: a review from the feedstocks to biodiesel production. Enzyme Res. https://doi.org/10.4061/2011/615803
Brígida AIS, Amaral PFF, Coelho MAZ, Gonçalves LRB (2014) Lipase from Yarrowia lipolytica: production, characterization and application as an industrial biocatalyst. J Mol Catal B Enzym 101:148–158. https://doi.org/10.1016/j.molcatb.2013.11.016
Fickers P, Nicaud JM, Gaillardin C et al (2004) Carbon and nitrogen sources modulate lipase production in the yeast Yarrowia lipolytica. J Appl Microbiol 96:742–749. https://doi.org/10.1111/j.1365-2672.2004.02190.x
Nunes PMB, Martins AB, Brígida AIS et al (2014) Intracellular lipase production by yarrowia lipolytica using different carbon sources. Chem Eng Trans 38:421–426. https://doi.org/10.3303/CET1438071
Iftikhar T, Abdullah R, Iqtedar M et al (2015) Production of lipases by Alternaria sp. (mbl 2810) through optimization of environmental conditions using submerged fermentation technique. Int J Biosci 7:178–186. https://doi.org/10.12692/ijb/7.2.178-186
Rywińska A, Witkowska D, Juszczyk P et al (2008) Waste frying oil as substrate for lipase production by Geotrichum candidum strains. Pol J Environ Stud 17:925–931
Moftah OAS, Grbavcic SZ, Moftah WAS et al (2013) Lipase production by Yarrowia lipolytica using olive oil processing wastes as substrates. J Serb Chem Soc 78:781–794. https://doi.org/10.2298/JSC120905005M
da Pereira A, Fontes-Santna GC, Amaral PFF (2019) Mango agro-industrial wastes for lipase production from Yarrowia lipolytica and the potential of the fermented solid as a biocatalyst. Food Bioprod Process 115:68–77. https://doi.org/10.1016/j.fbp.2019.02.002
Mateo C, Palomo JM, Fernandez-Lorente G et al (2007) Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb Technol 40:1451–1463. https://doi.org/10.1016/j.enzmictec.2007.01.018
Sheldon RA, van Pelt S (2013) Enzyme immobilisation in biocatalysis: why, what and how. Chem Soc Rev 42:6223–6235. https://doi.org/10.1039/c3cs60075k
Reis CLB, Sousa EYA, de Serpa J, F, et al (2019) Quim Nova. Quim Nova 42:768–783
Hanefeld U, Gardossi L, Magner E (2009) Understanding enzyme immobilisation. Chem Soc Rev 38:453–468. https://doi.org/10.1039/b711564b
Rodrigues RC, Ortiz C, Berenguer-Murcia Á et al (2013) Modifying enzyme activity and selectivity by immobilization. Chem Soc Rev 42:6290–6307. https://doi.org/10.1039/c2cs35231a
Fukuda H, Hama S, Tamalampudi S, Noda H (2008) Whole-cell biocatalysts for biodiesel fuel production. Trends Biotechnol 26:668–673. https://doi.org/10.1016/j.tibtech.2008.08.001
Barth G, Gaillardin C (1997) Physiology and genetics of the dimorphic fungus Yarrowia lipolytica. FEMS Microbiol Rev 19:219–237. https://doi.org/10.1016/S0168-6445(97)00002-8
Fickers P, Fudalej F, Le Dall MT et al (2005) Identification and characterisation of LIP7 and LIP8 genes encoding two extracellular triacylglycerol lipases in the yeast Yarrowia lipolytica. Fungal Genet Biol 42:264–274. https://doi.org/10.1016/j.fgb.2004.12.003
Ota Y, Gomi K, Kato S et al (1982) Purification and some properties of cell-bound lipase from Saccharomycopsis lipolytica. Agric Biol Chem 46:2885–2893. https://doi.org/10.1271/bbb1961.46.2885
Haba E, Bresco O, Ferrer C et al (2000) Isolation of lipase-secreting bacteria by deploying used frying oil as selective substrate. Enzyme Microb Technol 26:40–44. https://doi.org/10.1016/S0141-0229(99)00125-8
Goldsmith PD (2008) Economics of soybean production, marketing, and utilization. Soybeans Chem Prod Process Util. https://doi.org/10.1016/B978-1-893997-64-6.50008-1
United States Department of Agriculture (2019) Brazil Soybean Oil Production by Year. In: https://www.indexmundi.com/agriculture/?country=br&commodity=soybean-oil&graph=production
Yaakob Z, Mohammad M, Alherbawi M et al (2013) Overview of the production of biodiesel from Waste cooking oil. Renew Sustain Energy Rev 18:184–193. https://doi.org/10.1016/j.rser.2012.10.016
Supple B, Howard-Hildige R, Gonzalez-Gomez E, Leahy JJ (2002) The effect of steam treating waste cooking oil on the yield of methyl ester. JAOCS, J Am Oil Chem Soc 79:175–178. https://doi.org/10.1007/s11746-002-0454-1
Dizge N, Aydiner C, Imer DY et al (2009) Biodiesel production from sunflower, soybean, and waste cooking oils by transesterification using lipase immobilized onto a novel microporous polymer. Bioresour Technol 100:1983–1991. https://doi.org/10.1016/j.biortech.2008.10.008
Sinisterra JV (1992) Application of ultrasound to biotechnology: an overview. Ultrasonics 30:180–185. https://doi.org/10.1016/0041-624X(92)90070-3
Knorr D, Zenker M, Heinz V, Lee DU (2004) Applications and potential of ultrasonics in food processing. Trends Food Sci Technol 15:261–266. https://doi.org/10.1016/j.tifs.2003.12.001
Borthwick KAJ, Coakley WT, McDonnell MB et al (2005) Development of a novel compact sonicator for cell disruption. J Microbiol Methods 60:207–216. https://doi.org/10.1016/j.mimet.2004.09.012
Kapturowska AU, Stolarzewicz IA, Krzyczkowska J, Białecka-Florjańczyk E (2012) Studies on the lipolytic activity of sonicated enzymes from Yarrowia lipolytica. Ultrason Sonochem 19:186–191. https://doi.org/10.1016/j.ultsonch.2011.06.015
Huang G, Chen S, Dai C et al (2017) Effects of ultrasound on microbial growth and enzyme activity. Ultrason Sonochem 37:144–149. https://doi.org/10.1016/j.ultsonch.2016.12.018
Bansode SR, Rathod VK (2017) An investigation of lipase catalysed sonochemical synthesis: a review. Ultrason Sonochem 38:503–529. https://doi.org/10.1016/j.ultsonch.2017.02.028
Navarro López E, Robles Medina A, González Moreno PA et al (2016) Biodiesel production from Nannochloropsis gaditana lipids through transesterification catalyzed by Rhizopus oryzae lipase. Bioresour Technol 203:236–244. https://doi.org/10.1016/j.biortech.2015.12.036
Fraga JL, Penha ACB, da Pereira A et al (2018) Use of yarrowia lipolytica lipase immobilized in cell debris for the production of lipolyzed milk fat (Lmf). Int J Mol Sci. https://doi.org/10.3390/ijms19113413
Hagler AN, Mendonça-Hagler LC (1981) Yeasts from marine and estuarine waters with different levels of pollution in the state of Rio de Janeiro, Brazil. Appl Environ Microbiol 41:173–178. https://doi.org/10.1128/aem.41.1.173-178.1981
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Fabiszewska AU, Kotyrba D, Nowak D (2015) Assortment of carbon sources in medium for Yarrowia lipolytica lipase production: a statistical approach. Ann Microbiol 65:1495–1503. https://doi.org/10.1007/s13213-014-0988-7
Beopoulos A, Chardot T, Nicaud JM (2009) Yarrowia lipolytica: a model and a tool to understand the mechanisms implicated in lipid accumulation. Biochimie 91:692–696. https://doi.org/10.1016/j.biochi.2009.02.004
Pereira-Meirelles FV, Rocha-Leão MHM (2000) Lipase location in Yarrowia lipolytica cells. Biotechnol Lett 22:71–75. https://doi.org/10.1023/A:1005672731818
Corzo G, Revah S (1999) Production and characteristics of the lipase from Yarrowia lipolytica 681. Bioresour Technol 70:173–180. https://doi.org/10.1016/S0960-8524(99)00024-3
Ramos MJ, Fernández CM, Casas A et al (2009) Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour Technol 100:261–268. https://doi.org/10.1016/j.biortech.2008.06.039
Liu WS, Pan XX, Jia B et al (2010) Surface display of active lipases Lip7 and Lip8 from Yarrowia Lipolytica on Saccharomyces cerevisiae. Appl Microbiol Biotechnol 88:885–891. https://doi.org/10.1007/s00253-010-2782-1
Zhao H, Zheng L, Wang X et al (2011) Cloning, expression and characterization of a new lipase from Yarrowia lipolytica. Biotechnol Lett 33:2445–2452. https://doi.org/10.1007/s10529-011-0711-8
Kumari A, Gupta R (2012) Extracellular expression and characterization of thermostable lipases, LIP8, LIP14 and LIP18, from Yarrowia lipolytica. Biotechnol Lett 34:1733–1739. https://doi.org/10.1007/s10529-012-0958-8
da Pereira S, Diniz MM, De Jong G et al (2019) Chitosan-alginate beads as encapsulating agents for Yarrowia lipolytica lipase: Morphological, physico-chemical and kinetic characteristics. Int J Biol Macromol 139:621–630. https://doi.org/10.1016/j.ijbiomac.2019.08.009
Brígida IS, Amaral FF, Gonçalves RB et al (2013) Yarrowia lipolytica IMUFRJ 50682: lipase production in a multiphase bioreactor. Curr Biochem Eng 1:65–74. https://doi.org/10.2174/22127119113019990005
Carvalho T, Finotelli PV, Bonomo RCF et al (2017) Evaluating aqueous two-phase systems for Yarrowia lipolytica extracellular lipase purification. Process Biochem 53:259–266. https://doi.org/10.1016/j.procbio.2016.11.019
Akil E, Carvalho T, Bárea B et al (2016) Accessing regio-and typo-selectivity of Yarrowia lipolytica lipase in its free form and immobilized onto magnetic nanoparticles. Biochem Eng J 109:101–111. https://doi.org/10.1016/j.bej.2015.12.019
Funding
The financial support of CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Ministry of Education, Brazil) and WBI (Wallonie Bruxelles International, Belgium, Grant no. 154157) is greatly acknowledged. P. Amaral received a research fellowship from FAPERJ (Fundação Carlos Chagas Filho de Amparo à Pesquisa no Estado do Rio de Janeiro) (Grant number E-26/202.870/2015 BOLSA) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) (Grant number 308626/2019–2).
Author information
Authors and Affiliations
Contributions
Conceptualization: PFFA, PF; data curation: PFFA; formal analysis: PMBN; funding acquisition: PFFA, PF; investigation: RBR, PMBN, JLF; methodology: JLF, PFFA, AISB, PF; project administration: PFFA, PF; resources: PFFA, PF; software: JLF, PFFA; supervision: PFFA, AISB, MHM-L, PF; validation: PMBN; visualization: PMBN, PFFA; roles/writing—original draft: PMBN; writing—review & editing: PFFA, PF.
Corresponding author
Ethics declarations
Conflicts of interest
All authors declare no competing/ conflicts of interests. The funders had no decision on the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nunes, P.M.B., Fraga, J.L., Ratier, R.B. et al. Waste soybean frying oil for the production, extraction, and characterization of cell-wall-associated lipases from Yarrowia lipolytica. Bioprocess Biosyst Eng 44, 809–818 (2021). https://doi.org/10.1007/s00449-020-02489-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-020-02489-0