Abstract
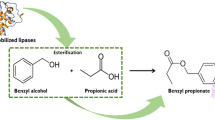
In this work, a fed-batch approach was adopted to overcome propionic acid lipase inactivation effects in the benzyl propionate direct esterification mediated by lipases. The ester synthesis was performed using commercial immobilized (Novozym 435) and lyophilized form Candida antarctica fraction B lipase (Cal B) as biocatalysts of the esterification between benzyl alcohol and propionic acid in a solvent-free system. The reaction involved the propionic acid-controlled addition during the first 5 h ensuring an excess of alcohol to dilute the media. The biocatalyst Novozym 435 showed a good performance in the first cycle of the fed-batch esterification, ensuring 90 and 99% of conversion at substrates molar ratio of 1:1 and 1:5 (acid:alcohol), respectively. However, the enzyme lost the activity and the conversions were sharply reduced at the second cycle. A novel qualitative protein content analysis by optical microscopy showed that the lipase was desorbed from the support after the esterification, and this behavior was strongly related to the presence of propionic acid in the reaction medium. The lyophilized Cal B was also tested as biocatalyst of the benzyl propionate esterification and showed a similar performance (related to the Novozym 435) in ester conversion and initial reaction rates for all substrates molar ratios tested. Since the substrates affected the performance of the Novozym 435, the lyophilized Cal B is the most suitable catalyst to the benzyl propionate esterification with conversions above 90%, considering a the fed-batch approach in a solvent-free system.





Similar content being viewed by others
References
Sá AGA, de Meneses AC, de Araújo PHH, de Oliveira D (2017) A review on enzymatic synthesis of aromatic esters used as flavor ingredients for food, cosmetics and pharmaceuticals industries. Trends Food Sci Technol. https://doi.org/10.1016/j.tifs.2017.09.004
de Meneses AC, Almeida Sá AG, Lerin LA et al (2019) Benzyl butyrate esterification mediated by immobilized lipases: evaluation of batch and fed-batch reactors to overcome lipase-acid deactivation. Process Biochem 78:50–57
Leszczak JP, Tran-Minh C (1998) Synthesis of benzoates by enzymatic catalysis in heterogeneous medium. J Mol Catal B Enzym 5:277–281. https://doi.org/10.1016/S1381-1177(98)00093-9
Tomke PD, Rathod VK (2015) Ultrasound assisted lipase catalyzed synthesis of cinnamyl acetate via transesterification reaction in a solvent free medium. Ultrason Sonochem 27:241–246. https://doi.org/10.1016/j.ultsonch.2015.04.022
Vanin AB, Orlando T, Piazza SP, Puton BMS (2014) Antimicrobial and antioxidant activities of clove essential oil and eugenyl acetate produced by enzymatic esterification. Appl Biochem Biotechnol 174:1286–1298. https://doi.org/10.1007/s12010-014-1113-x
Badgujar KC, Bhanage BM (2015) The combine use of ultrasound and lipase immobilized on co-polymer matrix for efficient biocatalytic application studies. J Mol Catal B Enzym 122:255–264. https://doi.org/10.1016/j.molcatb.2015.09.012
Gryglewicz S, Jadownicka E, Czerniak A (2000) Lipase catalysed synthesis of aliphatic, terpene and aromatic esters by alcoholysis in solvent-free medium. Biotechol Lett 22:1379–1382. https://doi.org/10.1023/A:1005600631600
Tischer W, Wedekind F (1999) Immobilized enzymes: methods and applications. Top Curr Chem 200:95–126. https://doi.org/10.1007/3-540-68116-7_4
Schmid A, Dordick JS, Hauer B et al (2001) Industrial biocatalysis today and tomorrow. Nature 409(6817):258–268
Sheldon RA (2007) Enzyme immobilization: the quest for optimum performance. Adv Synth Catal 349:1289–1307. https://doi.org/10.1002/adsc.200700082
Kasche V, Haufler U (1987) Equilibrium and kinetically controlled synthesis with enzymes: semisynthesis of penicillins and peptides. Methods Enzymol 136:280–292
Kasche V (1986) Mechanism and yields in enzyme catalysed equilibrium and kinetically controlled synthesis of fl-lactam antibiotics, peptides and other condensation products. Enzyme Microb Technol 8:4–16
Halling PJ (1990) High-affinity binding of water by proteins is similar in air and in organic solvents. Biochim Biophys Acta 1040:225–228
Halling PJ (1984) Effects of water on equilibria catalysed by hydrolytic enzymes in biphasic reaction systems. Enzyme Microb Technol 6:513–516
Wu Z, Qi W, Wang M et al (2014) Lipase immobilized on novel ceramic supporter with Ni activation for efficient cinnamyl acetate synthesis. J Mol Catal B Enzym 110:32–38. https://doi.org/10.1016/j.molcatb.2014.09.010
Hari Krishna S, Divakar S, Prapulla SG, Karanth NG (2001) Enzymatic synthesis of isoamyl acetate using immobilized lipase from Rhizomucor miehei. J Biotechnol 87:193–201. https://doi.org/10.1016/S0168-1656(00)00432-6
Hari Krishna S, Prapulla SG, Karanth NG (2000) Enzymatic synthesis of isoamyl butyrate using immobilized Rhizomucor miehei lipase in non-aqueous media. J Ind Microbiol Biotechnol 25:147–154. https://doi.org/10.1038/sj.jim.7000045
Claon PA, Akoh CC (1994) Effect of reaction parameters on SP435 lipase-catalyzed synthesis of Citronellyl acetate in organic solvent. Enzym Microb Technol 16:835–838. https://doi.org/10.1016/0141-0229(94)90056-6
Huang SY, Chang HL, Goto M (1998) Preparation of surfactant-coated lipase for the esterification of geraniol and acetic acid in organic solvents. Enzyme Microb Technol 0229:552–557
Marty A, Chulalaksananukul W, Willemot RM, Condoret JS (1991) Kinetics of lipase-catalyzed esterification in supercritical CO2. Biotechnol Bioeng 39:273–280
Khan NR, Rathod VK (2015) Enzyme catalyzed synthesis of cosmetic esters and its intensification: a review. Process Biochem 50:1793–1806. https://doi.org/10.1016/j.procbio.2015.07.014
Paludo N, Alves JS, Altmann C et al (2015) The combined use of ultrasound and molecular sieves improves the synthesis of ethyl butyrate catalyzed by immobilized Thermomyces lanuginosus lipase. Ultrason Sonochem 22:89–94. https://doi.org/10.1016/j.ultsonch.2014.05.004
Bansode SR, Rathod VK (2014) Ultrasound assisted lipase catalysed synthesis of isoamyl butyrate. Process Biochem 49:1297–1303. https://doi.org/10.1016/j.procbio.2014.04.018
Sekeroglu G, Fadiloglu S, Ibanoglu E (2002) Production and characterisation of enzymatically produced lauric acid esters of fructose. J Sci Food Agric 1522:1516–1522. https://doi.org/10.1002/jsfa.1222
Khan NR, Pratap AP (2013) Green synthesis of isopropyl ricinoleate. J Oleo Sci 158:153–158
McGinty D, Letizia CS, Api AM (2012) Fragrance material review on benzyl propionate. Food Chem Toxicol 50:S486–S490. https://doi.org/10.1016/j.fct.2013.08.067
VCF (2017) Volatile compounds in food: database. In: Nijssen LM, van Ingen-Visscher CA, Donders JJH (eds) Version 16.4. Triskelion B.V, Zeist, pp 1963–2017
Badgujar KC, Bhanage BM (2014) Enhanced biocatalytic activity of lipase immobilized on biodegradable copolymer of chitosan and polyvinyl alcohol support for synthesis of propionate ester: kinetic approach. Ind Eng Chem Res 53:18806–18815. https://doi.org/10.1021/ie501304e
Sá AGA, de Meneses AC, Lerin LA et al (2018) Biocatalysis of aromatic benzyl-propionate ester by different immobilized lipases. Bioprocess Biosyst Eng. https://doi.org/10.1007/s00449-018-1893-4
Güvenç A, Kapucu N, Mehmetoǧlu Ü (2002) The production of isoamyl acetate using immobilized lipases in a solvent-free system. Process Biochem 38:379–386. https://doi.org/10.1016/S0032-9592(02)00099-7
Romero MD, Calvo L, Alba C et al (2005) Enzymatic synthesis of isoamyl acetate with immobilized Candida antarctica lipase in n-hexane. Enzym Microb Technol 37:42–48. https://doi.org/10.1016/j.enzmictec.2004.12.033
Badgujar KC, Pai PA, Bhanage BM (2016) Enhanced biocatalytic activity of immobilized Pseudomonas cepacia lipase under sonicated condition. Bioprocess Biosyst Eng 39:211–221. https://doi.org/10.1007/s00449-015-1505-5
Zare M, Golmakani MT, Niakousari M (2019) Lipase synthesis of isoamyl acetate using different acyl donors: comparison of novel esterification techniques. Lwt 101:214–219. https://doi.org/10.1016/j.lwt.2018.10.098
Chiaradia V, Soares NS, Valério A et al (2016) Immobilization of Candida antarctica lipase B on magnetic poly(urea-urethane) nanoparticles. Appl Biochem Biotechnol 180:558–575. https://doi.org/10.1007/s12010-016-2116-6
Chen B, Miller ME, Gross RA (2007) Effects of porous polystyrene resin parameters on Candida antarctica lipase B adsorption, distribution, and polyester synthesis activity. Langmuir 23:6467–6474. https://doi.org/10.1021/la063515y
Zenevicz MCP, Jacques A, Furigo AF et al (2016) Enzymatic hydrolysis of soybean and waste cooking oils under ultrasound system. Ind Crops Prod 80:235–241. https://doi.org/10.1016/j.indcrop.2015.11.031
Ceni G, Lerin LA, de Conto JF et al (2010) Optimization of 1-glyceryl benzoate production by enzymatic transesterification in organic solvents. Enzym Microb Technol 46:107–112. https://doi.org/10.1016/j.enzmictec.2009.09.011
Hidayat C, Prastowo I, Hastuti P, Restiani R (2014) Effect of ethanol concentrations on rice bran protease activity and ester synthesis during enzymatic synthesis of oleic acid ethyl ester in a fed-batch system using crude rice bran (Oryza sativa) lipase. Biocatal Biotransform 32:231–235. https://doi.org/10.3109/10242422.2014.934683
Ortiz C, Ferreira ML, Barbosa O et al (2019) Novozym 435: the “perfect” lipase immobilized biocatalyst? Catal Sci Technol 9:2380–2420. https://doi.org/10.1039/c9cy00415g
Virgen-ortíz JJ, Tacias-pascacio VG, Hirata DB et al (2017) Relevance of substrates and products on the desorption of lipases physically adsorbed on hydrophobic supports. Enzym Microb Technol 96:30–35. https://doi.org/10.1016/j.enzmictec.2016.09.010
Rueda N, Albuquerque TL, Bartolome-cabrero R et al (2016) Reversible immobilization of lipases on heterofunctional octyl-amino agarose beads. Molecules 21:646. https://doi.org/10.3390/molecules21050646
Panahi Y, Akhavan A, Sahebkar A et al (2014) Investigation of the effectiveness of Syzygium aromaticum, Lavandula angustifolia and Geranium robertianum essential oils in the treatment of acute external otitis: a comparative trial with ciprofloxacin. J Microbiol Immunol Infect 47:211–216. https://doi.org/10.1016/j.jmii.2012.10.002
Rosso Comim SR, Veneral JG, De Oliveira D et al (2015) Enzymatic synthesis of poly(ε-caprolactone) in liquified petroleum gas and carbon dioxide. J Supercrit Fluids 96:334–348. https://doi.org/10.1016/j.supflu.2014.07.004
Gumel AM, Annuar SM, Heidelberg T (2013) Single-step lipase-catalyzed functionalization of medium-chain-length polyhydroxyalkanoates. J Chem Technol Biotechnol 88:1328–1335. https://doi.org/10.1002/jctb.3980
Polloni AE, Veneral JG, Rebelatto EA et al (2017) Enzymatic ring opening polymerization of ω-pentadecalactone using supercritical carbon dioxide. J Supercrit Fluids 119:221–228. https://doi.org/10.1016/j.supflu.2016.09.019
Rathore V, Madras G (2007) Synthesis of biodiesel from edible and non-edible oils in supercritical alcohols and enzymatic synthesis in supercritical carbon dioxide. Fuel 86:2650–2659. https://doi.org/10.1016/j.fuel.2007.03.014
Talukder MMR, Wu JC, Lau SK et al (2009) Comparison of Novozym 435 and Amberlyst 15 as heterogeneous catalyst for production of biodiesel from palm fatty acid distillate. Energy Fuels 23:1–4. https://doi.org/10.1021/ef8006245
Colombie S, Tweddell RJ, Condoret J-S, Marty A (1998) Water activity control: a way to improve the efficiency of continuous lipase esterification. Biotechnol Bioeng 60:362–368
Foresti ML, Pedernera M, Bucal V, Ferreira ML (2007) Multiple effects of water on solvent-free enzymatic esterifications. Enzyme Microb Technol 41:62–70. https://doi.org/10.1016/j.enzmictec.2006.11.023
Nie K, Xie F, Wang F, Tan T (2006) Lipase catalyzed methanolysis to produce biodiesel : optimization of the biodiesel production. J Mol Catal B Enzym 43:142–147. https://doi.org/10.1016/j.molcatb.2006.07.016
Poppe JK, Garcia-galan C, Matte CR et al (2013) Optimization of synthesis of fatty acid methyl esters catalyzed by lipase B from Candida antarctica immobilized on hydrophobic supports. J Mol Catal B Enzym 94:51–56. https://doi.org/10.1016/j.molcatb.2013.05.010
Abaházi E, Boros Z, Poppe L (2014) Additives enhancing the catalytic properties of lipase from Burkholderia cepacia immobilized on mixed-function-grafted mesoporous silica gel. Molecules 19:9818–9837. https://doi.org/10.3390/molecules19079818
Rueda N, Santos JCS, Torres R et al (2015) Improved performance of lipases immobilized on heterofunctional octyl-glyoxyl agarose beads. RSC Adv 5:11212–11222. https://doi.org/10.1039/c4ra13338b
Zaak H, Fernandez-lopez L, Otero C et al (2017) Improved stability of immobilized lipases via modification with polyethylenimine and glutaraldehyde. Enzym Microb Technol 106:67–74. https://doi.org/10.1016/j.enzmictec.2017.07.001
Filice GFM, Carolina DL, Lorena P et al (2011) Cross-linking of lipases adsorbed on hydrophobic supports: highly selective hydrolysis of fish oil catalyzed by RML. J Am Oil Chem Soc 88:801–807. https://doi.org/10.1007/s11746-010-1727-2
Barbosa O, Torres R, Ortiz C et al (2013) Heterofunctional supports in enzyme immobilization : from traditional immobilization protocols to opportunities in tuning enzyme properties. Biomacromolecules 14(8):2433–2462
Garcia-galan C, Fernandez-lafuente R, Rodrigues RC (2011) Potential of different enzyme immobilization strategies to improve enzyme performance. Adv Synth Catal 354:2885–2904. https://doi.org/10.1002/adsc.201100534
de Melo RR, Alnoch RC, Ferreira A et al (2017) New heterofunctional supports based on glutaraldehyde-activation: a tool for enzyme immobilization at neutral pH. Molecules 22(7):1088. https://doi.org/10.3390/molecules22071088
Arana-Peña S, Lokha Y, Fernández-Lafuente R (2018) Immobilization of eversa lipase on octyl agarose beads and preliminary characterization of stability and activity features. Catalysis. https://doi.org/10.3390/catal8110511
Hirata DB, Albuquerque TL, Rueda N et al (2016) Evaluation of different immobilized lipases in transesterification reactions using tributyrin: advantages of the heterofunctional octyl agarose beads. J Mol Catal B Enzym. https://doi.org/10.1016/j.molcatb.2016.08.008
Peirce S, Tacias-pascacio VG, Russo ME et al (2016) Stabilization of Candida antarctica lipase B (CALB) immobilized on octyl agarose by treatment with polyethyleneimine (PEI). Molecules. https://doi.org/10.3390/molecules21060751
Rueda N, Santos JCS, Torres R et al (2015) Improved performance of lipases immobilized on heterofunctional octyl-glyoxyl agarose beads. RSC Adv 5:11212–11222. https://doi.org/10.1039/c4ra13338b
Acknowledgements
The authors thank the financial support from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), and Laboratório Multiusuário de Estudos em Biologia from Universidade Federal de Santa Catarina (LAMEB/UFSC) for optical microscopy analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Meneses, A.C., Lerin, L.A., Araújo, P.H.H. et al. Benzyl propionate synthesis by fed-batch esterification using commercial immobilized and lyophilized Cal B lipase. Bioprocess Biosyst Eng 42, 1625–1634 (2019). https://doi.org/10.1007/s00449-019-02159-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-019-02159-w