Abstract
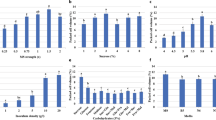
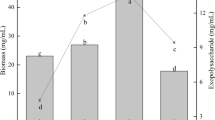
Natural products are gaining tremendous importance in pharmaceutical industry and attention has been focused on the applications of in vitro technologies to enhance yield and productivity of such products. In this study, we investigated the accumulation of biomass and antioxidant secondary metabolites in response to different carbohydrate sources (sucrose, maltose, fructose and glucose) and sucrose concentrations (1, 3, 5, 7 and 9 %). Moreover, the effects of 3 % repeated sucrose feeding (day-12, -18 and -24) were also investigated. The results showed the superiority of disaccharides over monosaccharides for maximum biomass and secondary metabolites accumulation. Comparable profiles for maximum biomass were observed in response to sucrose and maltose and initial sucrose concentrations of 3 and 5 %. Maximum total phenolic and total flavonoid contents were displayed by cultures treated with sucrose and maltose; however, initial sucrose concentrations of 5 and 7 % were optimum for both classes of metabolites, respectively. Following 3 % extra sucrose feeding, cultures fed on day-24 (late-log phase) showed higher biomass, total phenolic and total flavonoid contents as compared to control cultures. Highest antioxidant activity was exhibited by maltose-treated cultures. Moreover, sucrose-treated cultures displayed positive correlation of antioxidant activity with total phenolics and total flavonoids production. This work describes the stimulatory role of disaccharides and sucrose feeding strategy for higher accumulation of phenolics and flavonoids, which could be potentially scaled up to bioreactor level for the bulk production of these metabolites in suspension cultures of A. absinthium.



Similar content being viewed by others
References
Juteau F, Jerkovic I, Masotti V, Milos M, Mastelic J, Bessiere J-M, Viano J (2003) Composition and antimicrobial activity of the essential oil of Artemisia absinthium. Planta Med 69(02):158–161
Canadanovic-Brunet JM, Djilas SM, Cetkovic GS, Tumbas VT (2005) Free-radical scavenging activity of wormwood (Artemisia absinthium L.) extracts. J Sci Food Agric 85(2):265–272
Krebs S, Omer TN, Omer B (2010) Wormwood (Artemisia absinthium) suppresses tumour necrosis factor alpha and accelerates healing in patients with Crohn’s disease—a controlled clinical trial. Phytomedicine 17(5):305–309
Shafi G, Hasan TN, Syed NA, Al-Hazzani AA, Alshatwi AA, Jyothi A, Munshi A (2012) Artemisia absinthium (AA): a novel potential complementary and alternative medicine for breast cancer. Mol Biol Rep 39(7):7373–7379
Irshad S, Mannan A, Mirza B (2011) Antimalarial activity of three Pakistani medicinal plants. Pak J Pharm Sci 24(4):589–591
Mohammadian A, Moradkhani S, Ataei S, Shayesteh TH, Sedaghat M, Kheiripour N, Ranjbar A (2016) Antioxidative and hepatoprotective effects of hydroalcoholic extract of Artemisia absinthium L. in rat. J Herb Med Pharmacol 5(1):29–32
Nikhat S, Ahmad S, Akhtar J, Jamil S (2013) Phytochemical and ethnopharmacological perspective of Afsantin (Artemisia absinthium Linn.). Ann Phytomed 2(2):105–109
Reinke RA, King PJ, Victoria JG, McDougall BR, Ma G, Mao Y, Reinecke MG, Robinson WE (2002) Dicaffeoyltartaric acid analogues inhibit human immunodeficiency virus type 1 (HIV-1) integrase and HIV-1 replication at nontoxic concentrations. J Med Chem 45(17):3669–3683
Sun X, Linden J (1999) Shear stress effects on plant cell suspension cultures in a rotating wall vessel bioreactor. J Ind Microbiol Biotechnol 22(1):44–47
Shi L, Wang C, Zhou X, Zhang Y, Liu Y, Ma C (2013) Production of salidroside and tyrosol in cell suspension cultures of Rhodiola crenulata. Plant Cell Tissue Organ Cult 114(3):295–303
Kretzschmar FS, Oliveira CJ Jr, Braga MR (2007) Differential sugar uptake by cell suspension cultures of Rudgea jasminoides, a tropical woody Rubiaceae. Vitro Cell Dev Biol Plant 43(1):71–78
Baque MA, Elgirban A, Lee E-J, Paek K-Y (2012) Sucrose regulated enhanced induction of anthraquinone, phenolics, flavonoids biosynthesis and activities of antioxidant enzymes in adventitious root suspension cultures of Morinda citrifolia (L.). Acta Physiol Plant 34(2):405–415
Yang Y, He F, Yu L, Ji J, Wang Y (2009) Flavonoid accumulation in cell suspension cultures of Glycyrrhiza inflata Batal under optimizing conditions. Zeitschrift für Naturforschung C 64(1):68–71
Shimon-Kerner N, Mills D, Merchuk JC (2000) Sugar utilization and invertase activity in hairy-root and cell-suspension cultures of Symphytum officinale. Plant Cell Tissue Organ Cult 62(2):89–94
Slone JH, Buckhout TJ (1991) Sucrose-dependent H+ transport in plasma-membrane vesicles isolated from sugarbeet leaves (Beta vulgaris L.). Planta 183(4):584–589
Haissig BE (1982) Carbohydrate and amino acid concentrations during adventitious root primordium development in Pinus banksiana Lamb. cuttings. For Sci 28(4):813–821
Calamar A, De Klerk G-J (2002) Effect of sucrose on adventitious root regeneration in apple. Plant Cell Tissue Organ Cult 70(2):207–212
Cui X-H, Murthy HN, Wu C-H, Paek K-Y (2010) Sucrose-induced osmotic stress affects biomass, metabolite, and antioxidant levels in root suspension cultures of Hypericum perforatum L. Plant Cell Tissue Organ Cult 103(1):7–14
Koch K (1996) Carbohydrate-modulated gene expression in plants. Annu Rev Plant Biol 47(1):509–540
Rolland F, Moore B, Sheen J (2002) Sugar sensing and signaling in plants. Plant Cell 14(suppl 1):S185–S205
Solfanelli C, Poggi A, Loreti E, Alpi A, Perata P (2006) Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol 140(2):637–646
Wu C-H, Dewir YH, Hahn E-J, Paek K-Y (2006) Optimization of culturing conditions for the production of biomass and phenolics from adventitious roots of Echinacea angustifolia. J Plant Biol 49(3):193–199
Weathers P, DeJesus-Gonzalez L, Kim Y, Souret F, Towler M (2004) Alteration of biomass and artemisinin production in Artemisia annua hairy roots by media sterilization method and sugars. Plant Cell Rep 23(6):414–418
Ali M, Abbasi BH (2014) Light-induced fluctuations in biomass accumulation, secondary metabolites production and antioxidant activity in cell suspension cultures of Artemisia absinthium L. J Photochem Photobiol B 140:223–227
Ali M, Abbasi BH, Ali GS (2015) Elicitation of antioxidant secondary metabolites with jasmonates and gibberellic acid in cell suspension cultures of Artemisia absinthium L. Plant Cell Tissue Organ Cult 120:1099–1106
Ali M, Abbasi BH, Ihsan-ul-Haq (2013) Production of commercially important secondary metabolites and antioxidant activity in cell suspension cultures of Artemisia absinthium L. Ind Crops Prod 49:400–406
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497
Velioglu Y, Mazza G, Gao L, Oomah B (1998) Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem 46(10):4113–4117
Chang C-C, Yang MH, Wen HM, Chern JC (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10(3):178–182
Abbasi BH, Khan MA, Mahmood T, Ahmad M, Chaudhary MF, Khan MA (2010) Shoot regeneration and free-radical scavenging activity in Silybum marianum L. Plant Cell Tissue Organ Cult 101(3):371–376
Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57:675–709
Wang Y, Weathers P (2007) Sugars proportionately affect artemisinin production. Plant Cell Rep 26(7):1073–1081
Shinde AN, Malpathak N, Fulzele DP (2009) Studied enhancement strategies for phytoestrogens production in shake flasks by suspension culture of Psoralea corylifolia. Bioresour Technol 100(5):1833–1839
Karwasara VS, Dixit VK (2013) Culture medium optimization for camptothecin production in cell suspension cultures of Nothapodytes nimmoniana (J. Grah.) Mabberley. Plant Biotechnol Rep 7(3):357–369
Zha X-Q, Luo J-P, Jiang S-T, Wang J-H (2007) Enhancement of polysaccharide production in suspension cultures of protocorm-like bodies from Dendrobium huoshanense by optimization of medium compositions and feeding of sucrose. Process Biochem 42(3):344–351
Nagella P, Murthy HN (2010) Establishment of cell suspension cultures of Withania somnifera for the production of with anolide A. Bioresour Technol 101(17):6735–6739
Nigra H, Alvarez M, Giulietti A (1990) Effect of carbon and nitrogen sources on growth and solasodine production in batch suspension cultures of Solanum eleagnifolium Cav. Plant Cell Tissue Organ Cult 21(1):55–60
Morkunas I, Marczak Ł, Stachowiak J, Stobiecki M (2005) Sucrose-induced lupine defense against Fusarium oxysporum: sucrose-stimulated accumulation of isoflavonoids as a defense response of lupine to Fusarium oxysporum. Plant Physiol Biochem 43(4):363–373
Benavides M (1997) Growth and thiophene accumulation are influenced by sucrose, light and subculture in cell suspensions and root cultures of Tagetes argentina. Biocell 21(1):1–11
Zhong J-J, Yoshida T (1995) High-density cultivation of Perilla frutescens cell suspensions for anthocyanin production: effects of sucrose concentration and inoculum size. Enzyme Microbial Technol 17(12):1073–1079
Wang H-Q, Zhong J-J, Yu J-T (1997) Enhanced production of taxol in suspension cultures of Taxus chinensis by controlling inoculum size. Biotechnol Lett 19(4):353–356
Wang H, Yu J, Zhong J (1999) Significant improvement of taxane production in suspension cultures of Taxus chinensis by sucrose feeding strategy. Process Biochem 35(5):479–483
Hakkim FL, Kalyani S, Essa M, Girija S, Song H (2011) Production of rosmarinic in Ocimum sanctum cell cultures by the influence of sucrose, phenylalanine, yeast extract, and methyl jasmonate. Int J Biol Med Res 2(4):1070–1074
Tian HQ, Russell SD (1998) Culture-induced changes in osmolality of tobacco cell suspensions using four exogenous sugars. Plant Cell Tissue Organ Cult 55(1):9–13
Fazal H, Abbasi BH, Ahmad N, Ali M, Ali S (2015) Sucrose induced osmotic stress and photoperiod regimes enhanced the biomass and production of antioxidant secondary metabolites in shake-flask suspension cultures of Prunella vulgaris L. Plant Cell Tissue Organ Cult 124(3):573–581
Suan See K, Bhatt A, Lai Keng C (2011) Effect of sucrose and methyl jasmonate on biomass and anthocyanin production in cell suspension culture of Melastoma malabathricum (Melastomaceae). Rev Biol Trop 59(2):597–606
Skrzypczak-Pietraszek E, Słota J, Pietraszek J (2014) The influence of l-phenylalanine, methyl jasmonate and sucrose concentration on the accumulation of phenolic acids in Exacum affine Balf. f. ex Regel shoot culture. Acta Biochim Pol 61(1):47–53
Kikowska M, Budzianowski J, Krawczyk A, Thiem B (2012) Accumulation of rosmarinic, chlorogenic and caffeic acids in in vitro cultures of Eryngium planum L. Acta Physiol Plant 34(6):2425–2433
Y-h Zhang, Zhong J-j J-j, Yu J-t (1996) Enhancement of ginseng saponin production in suspension cultures of Panax notoginseng: manipulation of medium sucrose. J Biotechnol 51(1):49–56
Kim J-H, Yun J-H, Hwang Y-S, Byun SY, Kim D-I (1995) Production of taxol and related taxanes in Taxus brevifolia cell cultures: effect of sugar. Biotechnol Lett 17(1):101–106
Shohael A, Chakrabarty D, Ali M, Yu K, Hahn E, Lee H, Paek K (2006) Enhancement of eleutherosides production in embryogenic cultures of Eleutherococcus sessiliflorus in response to sucrose-induced osmotic stress. Process Biochem 41(3):512–518
Wang C, Wu J, Mei X (2001) Enhanced taxol production and release in Taxus chinensis cell suspension cultures with selected organic solvents and sucrose feeding. Biotechnol Prog 17(1):89–94
Asghar MN, Khan IU, Bano N (2011) In vitro antioxidant and radical-scavenging capacities of Citrullus colocynthes (L) and Artemisia absinthium extracts using promethazine hydrochloride radical cation and contemporary assays. Food Sci Technol Int 17(5):481–494
Craciunescu O, Constantin D, Gaspar A, Toma L, Utoiu E, Moldovan L (2012) Evaluation of antioxidant and cytoprotective activities of Arnica montana L. and Artemisia absinthium L. ethanolic extracts. Chem Cent J 6(1):1–11
Lopes-Lutz D, Alviano DS, Alviano CS, Kolodziejczyk PP (2008) Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry 69(8):1732–1738
Mahmoudi M, Ebrahimzadeh M, Ansaroudi F, Nabavi S, Nabavi S (2009) Antidepressant and antioxidant activities of Artemisia absinthium L. at flowering stage. Afr J Biotechnol 8(24):7170–7175
Taherkhani M, Rustaiyan A, Rasooli I, Taherkhani T (2013) Chemical composition, antimicrobial activity, antioxidant and total phenolic content within the leaves essential oil of Artemisia absinthium L. growing wild in Iran. Afr J Pharm Pharmacol 7(2):30–36
Lee Y-J, Thiruvengadam M, Chung I-M, Nagella P (2013) Polyphenol composition and antioxidant activity from the vegetable plant Artemisia absinthium L. Aust J Crop Sci 7(12):1921–1926
Acknowledgments
We are thankful to University Research Fund of Quaid-i-Azam University, Pakistan for providing us financial support to accomplish this research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ali, M., Abbasi, B.H., Ahmad, N. et al. Sucrose-enhanced biosynthesis of medicinally important antioxidant secondary metabolites in cell suspension cultures of Artemisia absinthium L.. Bioprocess Biosyst Eng 39, 1945–1954 (2016). https://doi.org/10.1007/s00449-016-1668-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-016-1668-8