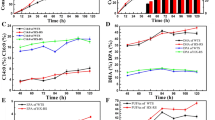
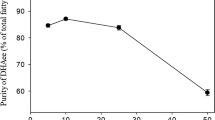
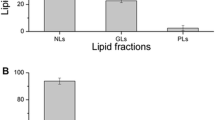
Abstract
Polyunsaturated fatty acids (PUFAs) are valuable ingredients in the food and pharmaceutical products due to their beneficial influence on human health. Most studies paid attention on the production of PUFAs from oleaginous micro-organisms but seldom on the comparative proteomics of cells. In the study, three methods (i.e., cold shock, acetone precipitation and ethanol precipitation) for lipid removal from crude protein extracts were applied in different PUFAs-producing micro-organisms. Among the selective strains, Schizochytrium was used as an oleaginous strain with high lipid of 60.3 (w/w %) in biomass. The Mortierella alpina and Cunninghamella echinulata were chosen as the low-lipid-content strains with 25.8 (w/w %) and 21.8 (w/w %) of lipid in biomass, respectively. The cold shock resulted as the most effective method for lipid removed, thus obtained higher protein amount for Schizochytrium. Moreover, from the comparative proteomics for the three PUFAs-producing strains, it showed more significant proteins of up or down-regulation were explored under cold shock treatment. Therefore, the essential proteins (i.e., polyunsaturated fatty acid synthase) and regulating proteins were observed. In conclusion, this study provides a valuable and practical approach for analysis of high PUFAs-producing strains at the proteomics level, and would further accelerate the understanding of the metabolic flux in oleaginous micro-organisms.





Similar content being viewed by others
References
Gill I, Valivety R (1997) Polyunsaturated fatty acids, part 1: occurrence, biological activities and applications. Trends Biotechnol 15:401–409
Sijtsma L, de Swaaf ME (2004) Biotechnological production and applications of the ω-3 polyunsaturated fatty acid docosahexaenoic acid. Appl Microbiol Biotechnol 64:146–153
de Swaaf ME, Sijtsma L, Pronk JT (2003) High-cell-density fed-batch cultivation of the docosahexaenoic acid producing marine alga Crypthecodinium cohnii. Biotechnol Bioeng 81:666–672
Ratledge C (2004) Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochimie 86:807–815
Hwang BH, Kim JW, Park CY, Park CS, Kim YS, Ryul YW (2005) High-level production of arachidonic acid by fed-batch culture of Mortierella alpina using NH4OH as a nitrogen source and pH control. Biotech Lett 27:731–735
Gema H, Kavadia A, Dimou D, Tsagou V, Komaitis M, Aggelis G (2002) Production of γ-linolenic acid by Cunninghamella echinulata cultivated on glucose and orange peel. Appl Microbiol Biotechnol 58:303–307
Papanikolaou S, Sarantou S, Komaitis M, Aggelis G (2004) Repression of reserve lipid turnover in Cunninghamella echinulata and Mortierella isabellina cultivated in multiple-limited media. J Appl Microbiol 97:867–875
Tchakouteu SS, Chatzifragkou A, Kalantzi O, Koutinas AA, Aggelis G, Papanikolaou S (2015) Oleaginous yeast Cryptococcus curvatus exhibits interplay between biosynthesis of intracellular sugars and lipids. Eur J Lipid Sci Technol 117:657–672
Bellou S, Makri A, Triantaphyllidou IE, Papanikolaou S, Aggelis G (2012) Lipids containing polyunsaturated fatty acids synthesized by Zygomycetes grown on glycerol. Appl Biochem Biotechnol 166:146–158
Ratledge C (2002) Regulation of lipid accumulation in oleaginous micro-organisms. Biochem Soc Trans 30:1047–1050
Chang GF, Luo ZL, Gu ST, Wu QH, Chang M, Wang XG (2013) Fatty acid shifts and metabolic activity changes of Schizochytrium sp. S31 cultured on glycerol. Bioresour Technol 142:255–260
Fakas S, Galiotou-Panayotou M, Papanikolaou S, Komaitis M, Aggelis G (2007) Compositional shifts in lipid fractions during lipid turnover in Cunninghamella echinulata. Enzyme Microb Technol 40:1321–1327
Ng IS, Ye CM, Zhang ZX, Lu YH, Jing KJ (2014) Daptomycin antibiotic production processes in fed-batch fermentation by Streptomyces roseosporus NRRL11379 with precursor effect and medium optimization. Bioprocess Biosyst Eng 37:415–423
Ng IS, Zheng XS, Chen BY, Chi XQ, Lu YH, Chang CS (2013) Proteomics approach to decipher novel genes and enzymes characterization of a bioelectricity-generating and dye-decolorizing bacterium Proteus hauseri ZMd44. Biotechnol Bioprocess Eng 18:8–17
Jiang L, He L, Fountoulakis M (2004) Comparison of protein precipitation methods for sample preparation prior to proteomic analysis. J Chromatogr A 1023:317–320
Wu XL, Gong FP, Wang W (2014) Protein extraction from plant tissues for 2DE and its application in proteomic analysis. Proteomics 14:645–658
Bodzon-Kulakowska A, Bierczynska-Krzysik A, Dylag T, Drabik A, Suder P, Noga M, Jarzebinska J, Silberring J (2007) Methods for samples preparation in proteomic research. J Chromatogr B 849:1–31
Wang W, Vignani R, Scali M, Sensi E, Tiberi E (2004) Removal of lipid contaminants by organic solvents from oilseed protein extract prior to electrophoresis. Anal Biochem 329:139–141
Shi JH, Feng HX, Lee J, Chen WN (2013) Comparative proteomics profile of lipid-cumulating oleaginous yeast: an iTRAQ-coupled 2-D LC-MS/MS analysis. PLoS ONE 8:e85532
Ren LJ, Huang H, Xiao AH, Lian M, Jin LJ, Ji XJ (2009) Enhanced docosahexaenoic acid production by reinforcing acetyl-CoA and NADPH supply in Schizochytrium sp. HX-308. Bioprocess Biosyst Eng 32:837–843
Jin JM, Huang H, Xiao AH, Zhang K, Liu X, Li S, Peng C (2008) A novel two-step fermentation process for improved arachidonic acid production by Mortierella alpine. Biotech Lett 30:1087–1091
Kavadia A, Komaitis M, Chevalot I, Blanchard F, Marc I, Aggelis G (2001) Lipid and γ-linolenic acid accumulation in strains of Zygomycetes growing on glucose. J Am Oil Chem Soc 78:341–346
Ling XP, Guo J, Liu XT, Zhang X, Wang N, Lu YH, Ng IS (2015) Impact of carbon and nitrogen feeding strategy on high production of biomass and docosahexaenoic acid (DHA) by Schizochytrium sp. LU310. Bioresour Technol 184:139–147
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Donnini S, Prinsi B, Negri AS, Vigani G, Espen L, Zocchi G (2010) Proteomic characterization of iron deficiency responses in Cucumis sativus L. roots. BMC Plant Biol 10:268–283
Nie ZK, Deng ZT, Zhang AH, Ji XJ, Huang H (2014) Efficient arachidonic acid-rich oil production by Mortierella alpina through a three-stage fermentation strategy. Bioprocess Biosyst Eng 37:505–511
Malik NSA, Bradford JM (2005) A simple protein extraction method for proteomic studies on olive leaves. J Food Agric Environ 3:246–248
Hurkman WJ, Tanaka CK (1986) Solubilization of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol 81:802–806
Natarajan S, Xu C, Caperna TJ, Garrett WM (2005) Comparison of protein solubilization methods suitable for proteomic analysis of soybean seed proteins. Anal Biochem 342:214–220
Joo WA, Lee DY, Kim CW (2003) Development of an effective sample preparation method for the proteome analysis of body fluids using 2-D gel electrophoresis. Biosci Biotechnol Biochem 67:1574–1577
Watkins LK, Bondarenko PV, Barbacci DC, Song S, Cockrill SL, Russell DH, Macfarlane RD (1999) Fast C18 solid-phase desalting/delipidation of the human serum apolipoproteins for matrix-assisted laser desorption ionization and electrospray ionization mass spectrometric analysis. J Chromatogr A 840:183–193
Schweizer E, Hofmann J (2004) Microbial type I fatty acid synthases (FAS): major players in a network of cellular FAS systems. Microbiol Mol Biol Rev 68:501–517
Hauvermalea A, Kunera J, Rosenzweiga B, Guerrab D, Diltza S, Metza JG (2006) Fatty acid production in Schizochytrium sp.: involvement of a polyunsaturated fatty acid synthase and a type I fatty acid synthase. Lipids 41:739–747
Acknowledgments
The authors are grateful to the National Natural Science Foundation of China (No. 41206115), the Natural Science Foundation of Fujian Province of China (No. 2013J01060) and the National High-Tech R&D Program of China (No. 2014AA021701).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Ling, X., Guo, J., Zheng, C. et al. Simple, effective protein extraction method and proteomics analysis from polyunsaturated fatty acids-producing micro-organisms. Bioprocess Biosyst Eng 38, 2331–2341 (2015). https://doi.org/10.1007/s00449-015-1467-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-015-1467-7