Abstract
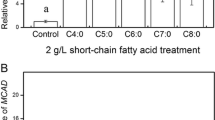
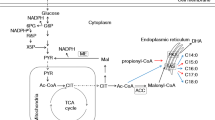
Schizochytrium sp. is a marine microalga that has been developed as a commercial source for docosahexaenoic acid (DHA, C22∶6 ω−3), enriched biomass, and oil. Previous work suggested that the DHA, as well as docosapentaenoic acid (DPA, C22∶5 ω−6), that accumulate in Schizochytrium are products of a multi-subunit polyunsaturated fatty acid (PUFA) synthase (1). Here we show data to support this view and also provide information of other aspects of fatty acid synthesis in this organism. Three genes encoding subunits of the PUFA synthase were isolated from genomic DNA and expressed in E. coli along with an essential accessory gene encoding a phosphopantetheinyl transferase (PPTase). The resulting transformants accumulated both DHA and DPA. The ratio of DHA to DPA was approximately the same as that observed in Schizochytrium. Treatment of Schizochytrium cells with certain levels of cerulenin resulted in inhibition of 14C acetate incorporation into short chain fatty acids without affecting labeling of PUFAs, indicating distinct biosynthetic pathways. A single large gene encoding the presumed short chain fatty acid synthase (FAS) was cloned and sequenced. Based on sequence homology and domain organization, the Schizochytrium FAS resembles a fusion of fungal FAS β and α subunits.
Similar content being viewed by others
Abbreviations
- ACP:
-
acyl carrier protein
- ARA:
-
arachidonic acid (C20∶4 ω−6)
- bp:
-
nucleotide base pair
- DHA:
-
docosahexaenoic acid (C22∶6 ω−3)
- DPA:
-
docosapentaenoic acid (C22∶5, this study refers exclusively to DPA ω−6)
- EPA:
-
eicosapentaenoic acid (C20∶5 ω−3)
- IPTG:
-
isopropyl-1-thio-β-D-galactopyranoside
- KAS:
-
β-ketoacyl-ACP synthase
- LC-PUFA:
-
those PUFA with carbon chain lengths greater than 18
- Orf:
-
open reading frame
- Orf B* :
-
Schizochytrium PUFA synthase Orf B modified for expression in E. coli
- PKS:
-
polyketide synthase
- PPTase:
-
phosphopantetheinyl transferase
- RBS:
-
ribosome binding site
References
Metz, J.G., Roessler, P., Facciotti, D., Levering, C., Dittrich, F., Lassner, M., Valentine, R., Lardizabal, K., Domergue, F., Yamada, A., Yazawa, K., Knauf, V., and Browse, J. (2001) Production of polyunsaturated fatty acids by polyketide synthases in both prokaryotes and eukaryotes. Science 293, 290–293.
Simopoulos, A.P. (1999) Essential fatty acids in health and chronic disease. Am. J. Clin. Nutr.. 70, 560S-569S.
Jump, D.B. (2002) The biochemistry of n−3 polyunsaturated fatty acids. J. Biol. Chem. 277, 8755–8758.
Jacobs, N.M., Covaci, A., Gheorghe, A., and Schepens, P. (2004) Time trend investigation of PCBs, PBDEs, and organochlorine pesticides in selected n−3 polyunsaturated fatty acid rich dietary fish oil and vegetable oil supplements; nutritional relevance for human essential n−3 fatty acid requirements. J. Agric. Food Chem. 52, 1780–1788.
Napier, J.A., Sayanova, O., Qi, B. and Lazarus, C.M. (2004) Progress toward the production of long-chain polyunsaturated fatty acids in transgenic plants. Lipids 39, 1067–1075.
Wu, G., Truksa, M., Datla, N., Vrinten, P., Bauer, J., Zank, T., Cirpus, P., Heinz, E., and Qiu, X. (2005) Stepwise engineering to produce high yields of very long-chain polyunsaturated fatty acids in plants. Nat. Biotechnol. 23, 1013–1017.
Kinney, A.J., Cahoon, E.B., Damude, H.G., Hitz, W.D., Liu, Z.-B., and Kolar, C.W. (2004) Production of very long chain polyunsaturated fatty acids in oilseed plants. United States Patent Application Publication. Pub. No.: US 2004/0172682 A1.
Domergue, F., Abbadi, A., Ott, C., Zank, T.K., Zahringer, U., and Heinz, E. (2003) Acyl carriers used as substrates by the desaturases and elongases involved in very long-chain polyunsaturated fatty acids biosynthesis reconstituted in yeast. J. Biol. Chem. 278, 35115–35126.
Streekstra, H. (2005) Arachidonic acid: fermentative production by Mortierella fungi, in Single Cell Oils, Cohen, Z. and Ratledge, C., eds., pp. 73–85, AOCS Press.
Wynn, J., Behrens, P., Sundararajan, A., Hansen, J., and Apt, K. (2005) Production of single cell oils by Dinoflagellates, in Single Cell Oils, Cohen, Z. and Ratledge, C., eds., pp. 86–98, AOCS Press.
Barclay, W., Weaver, C., and Metz, J. (2005) Development of a docosahexaenoic acid production technology using Schizochytrium: A historical perspective, in Single Cell Oils, Cohen, Z. and Ratledge, C., eds., pp. 36–52, AOCS Press.
DeLong, E. and Yayanos, A.A. (1986) Biochemical function and ecological significance of novel bacterial lipids in deep-sea prokaryotes. Applied and Environmental Microbiology 51, 730–737
Yazawa, K. (1996) Production of eicosapentaenoic acid from marine bacteria. Lipids 31, S297-S300.
Yu, R., Yamada, A., Watanabe, K., Yazawa, K., Takeyama, H., Matsunaga, T., and Kurane, R. (2000) Production of eicosapentaenoic acid by recombinant marine cyanobacterium, Synechococcus sp. Lipids 35, 1061–1064.
Lambalot, R.H., Gehring, A.H., Flugel, R.S., Zuber, P., LaCele, M., Marahiel, M.A., Khosla, C., and Walsh, C.T. (1996) A new enzyme superfamily-the phosphopantetheinyl transferases. Chemistry & Biology 3, 923–936.
Facciotti, D., Metz, J.G., and Lassner, M. (2000) Production of polyunsaturated fatty acids by expression of polyketide-like synthesis genes in plants. U.S. Patent 6,140,486.
Kaulmann, U. and Hertweck, C. (2002) Biosynthesis of polyunsaturated fatty acids by polyketide synthases. Angew. Chem. Int. Ed. 41, 1866–1869.
Orikasa, Y., Nishida, T., Hase, A., Watanabe, K., Morita, N., and Okuyama, H. (2006) A phosphopantetheinyl transferase gene essential for biosynthesis of n−3 polyunsaturated fatty acids from Moritella marina strain MP-1. FEBS Letters 580, 4423–4429.
Pikaart, M.J. and Felsenfeld, G. (1996) Expression and codon usage optimization of the erythroid-specific transcription factor cGATA-1 in baculoviral and bacterial systems. Protein Expr. Purif. 8, 469–475.
Nakano, M.M., Corbell, N., Besson, J., and Zuber, P. (1992) Isolation and characterization of sfp: a gene that functions in the production of the lipopeptide biosurfactant, surfactin, in Bacillus subtilis. Mol. Gen. Genet. 232, 313–321.
Black, T.A. and Wolk, C.P. (1994) Analysis of a Het− mutation in Anabaena sp. strain PCC 7120 implicates a secondary metabolite in the regulation of heterocyst spacing. Journal of Bacteriology 176, 2282–2292.
Yang, Y.M., Zhang, J.Y., and Huang, Z.H. (1989) Combined inbeam impact-B/E-linked scan mass spectrometry of oxazoline derivatives for the structure determination of long-chain unsaturated fatty acids. J. of Lipid Research 30, 127–133.
Ashford, A., Barclay, W.R., Weaver, C.A., Giddings, T.H., and Zeller, S. (2000) Electron microscopy may reveal structure of docosahexaenoic acid-rich oil within Schizochytrium sp. Lipids 35, 1377–1386.
Campbell, E.L., Cohen, M.F., and Meeks, J.C. (1997) A polyketide-synthase-like gene is involved in the synthesis of heterocyst glycolipids in Nostoc punctiforme strain ATCC 29133. Arch. Microbiol. 167, 251–258.
Cavalier-Smith, T., Allsopp, M.T.E.P., and Chao, E.E. (1994) Thraustochytrids are chromists not fungi: 18sRNA signatures of Heterokonta. Philos. Trans. R. Soc. London B: Biol. Sci. 346, 387–397.
Qiu, X., Hong, H., and MacKenzie, S.L. (2001) Identification of a Δ4 fatty acid desaturase from Thraustochytrium sp. involved in the biosynthesis of docosahexaenoic acid by heterologous expression in Saccharomyces cerevisiae and Brassica juncea. J. Biol. Chem. 276, 31561–31566.
Omura, S. (1976) The antibiotic cerulenin, a novel tool for biochemistry as an inhibitor of fatty acid synthesis. Bacteriological reviews 40, 681–697.
Morita, N., Nishida, T., Tanaka, M., Yano, Y., and Okuyama, H. (2005) Enhancement of polyunsaturated fatty acid production by cerulenin treatment in polyunsaturated fatty acid-producing bacteria. Biotechnol. Lett. 27, 389–393.
Schweizer, E. and Hofmann, J. (2004) Microbial type I fatty acid synthases (FAS): Major players in a network of cellular FAS systems. Microbio. Mol. Biol. Reviews 68, 501–517
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hauvermale, A., Kuner, J., Rosenzweig, B. et al. Fatty acid production in Schizochytrium sp.: Involvement of a polyunsaturated fatty acid synthase and a type I fatty acid synthase. Lipids 41, 739–747 (2006). https://doi.org/10.1007/s11745-006-5025-6
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11745-006-5025-6