Abstract
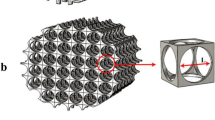
Currently, RWVB (Rotating wall vessel bioreactor) combined with a microcarrier used for in vitro expansion revealed that the suspended cells attached on the microcarrier will collide with outer and inner cylinders of RWVB inevitably, which leads to harmful results to the cells. Considering this, hollow fiber (HF) membrane module treated as a cell carrier is adopted to combine with RWVB to form a novel rotating wall hollow fiber membrane bioreactor (RWHMB) to avoid aforementioned harmful collision, since the cells cultured inside this bioreactor will mainly adhere to large specific surface of hollow fiber membrane module. Prior to cell experiment, mathematical simulations concerned with flow field inside RWHMB are performed by CFD, which includes the distributions of the total pressure, velocity, and shear stress with the variation of rotating speeds and directions, as well as the radial location and diameter of hollow fiber membrane. To further confirm the feasible parameters getting from the simulation, this RWHMB is adopted to expand osteoblasts isolated from SD rats within its dynamic conditions. Cell expansion in T-flask is carried out as a negative control. The results showed that with the same rotating direction and speed of 10 rpm, inner and outer cylinders of RWHMB generated cyclical stress stimulus, which was acceptable to cell expansion and facilitated the secretion of extracellular matrix. Besides, hollow fiber membrane carrier with a diameter of 0.2 mm has an excellent biocompatibility and their radial locations presented a tiny influence on flow field inside the culture chamber.
















Similar content being viewed by others
References
Ripamonti U, Duarte R, Ferretti C (2014) Re-evaluating the induction of bone formation in primates. Biomaterials 35(35):9407–9422
Moll G, Alm JJ, Davies LC, von Bahr L, Heldring N, Stenbeck-Funke L, Hamad OA, Hinsch R, Ignatowicz L, Locke M, Lönnies H, Lambris JD, Teramura Y, Nilsson-Ekdahl K, Nilsson B, Le Blanc K (2014) Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties? Stem Cells 32(9):2430–2442
Kedong S, Wenfang L, Yanxia Z, Hong W, Ze Y, Mayasari L, Tianqing L (2014) Dynamic fabrication of tissue-engineered bone substitutes based on derived cancellous bone scaffold in a spinner flask bioreactor system. Appl Biochem Biotechnol 174(4):1331–1343
Miklas JW, Nunes SS, Sofla A, Reis LA, Pahnke A, Xiao Y, Laschinger C, Radisic M (2014) Bioreactor for modulation of cardiac microtissue phenotype by combined static stretch and electrical stimulation. Biofabrication 6(2):024113
Dos Santos F, Campbell A, Fernandes-Platzgummer A, Andrade PZ, Gimble JM, Wen Y, Boucher S, Vemuri MC, da Silva CL, Cabral JM (2014) A xenogeneic-free bioreactor system for the clinical-scale expansion of human mesenchymal stem/stromal cells. Biotechnol Bioeng 111(6):1116–1127
Mahler GJ, Frendl CM, Cao Q, Butcher JT (2014) Effects of shear stress pattern and magnitude on mesenchymal transformation and invasion of aortic valve endothelial cells. Biotechnol Bioeng 111(11):2326–2337
Vozzi F, Bianchi F, Ahluwalia A, Domenici C (2014) Hydrostatic pressure and shear stress affect endothelin-1 and nitric oxide release by endothelial cells in bioreactors. Biotechnol J 9(1):146–154
Yeatts AB, Choquette DT, Fisher JP (2013) Bioreactors to influence stem cell fate: augmentation of mesenchymal stem cell signaling pathways via dynamic culture systems. Biochim Biophys Acta 1830(2):2470–2480
Li Y, Luo Y, Xie Z, Xing J, Lin M, Yang L, Wang Y, Huang K (2013) The optimal combination of substrate chemistry with physiological fluid shear stress. Colloids Surf B Biointerfaces 112:51–60
Maul TM, Chew DW, Nieponice A, Vorp DA (2011) Mechanical stimuli differentially control stem cell behavior: morphology, proliferation, and differentiation. Biomech Model Mechanobiol 10(6):939–953
Bilgen B, Chu D, Stefani R, Aaron RK (2013) Design of a biaxial mechanical loading bioreactor for tissue engineering. J Vis Exp (74):e50387
Stoddart MJ, Ettinger L, Häuselmann HJ (2006) Enhanced matrix synthesis in de novo, scaffold free cartilage-like tissue subjected to compression and shear. Biotechnol Bioeng 95(6):1043–1051
Zhang ZY, Teoh SH, Teo EY, Khoon Chong MS, Shin CW, Tien FT, Choolani MA, Chan JK (2010) A comparison of bioreactors for culture of fetal mesenchymal stem cells for bone tissue engineering. Biomaterials 31(33):8684–8695
Song K, Liu T, Cui Z, Li X, Ma X (2008) Three-dimensional fabrication of engineered bone with human bio-derived bone scaffolds in a rotating wall vessel bioreactor. J Biomed Mater Res A 86(2):323–332
Skardal A, Sarker SF, Crabbé A, Nickerson CA, Prestwich GD (2010) The generation of 3-D tissue models based on hyaluronan hydrogel-coated microcarriers within arotatingwall vessel bioreactor. Biomaterials 31(32):8426–8435
Song K, Yang Z, Liu T, Zhi W, Li X, Deng L, Cui Z, Ma X (2006) Fabrication and detection of tissue-engineered bones with bio-derived scaffolds in a rotating bioreactor. Biotechnol Appl Biochem 45(Pt 2):65–74
Gharravi AM, Orazizadeh M, Ansari-Asl K, Banoni S, Izadi S, Hashemitabar M (2012) Design and fabrication of anatomical bioreactor systems containing alginate scaffolds for cartilage tissue engineering. Avicenna J Med Biotechnol. 4(2):65–74
Bilgen B, Barabino GA (2012) Modeling of bioreactor hydrodynamic environment and its effects on tissue growth. Methods Mol Biol 868:237–255
Song K, Wang H, Zhang B, Lim M, Liu Y, Liu T (2013) Numerical simulation of fluid field and in vitro three-dimensional fabrication of tissue-engineered bones in a rotating bioreactor and in vivo implantation for repairing segmental bone defects. Cell Stress Chaperones 18(2):193–201
Fan Y, Hsiung M, Cheng C, Tzanakakis ES (2014) Facile engineering of xeno-free microcarriers for the scalable cultivation of human pluripotent stem cells in stirred suspension. Tissue Eng Part A 20(3–4):588–599
Lock LT, Tzanakakis ES (2009) Expansion and differentiation of human embryonic stem cells to endoderm progeny in a micro carrier stirred-suspension culture. Tissue Eng Part A 15(8):2051–2056
Granet C, Laroche N, Vico L, Alexandre C, Lafage-Proust MH (1998) Rotating-wall vessels, promising bioreactors for osteoblastic cell culture: comparison with other 3D conditions. Med Biol Eng Comput 36(4):513–519
Meyers VE, Zayzafoon M, Douglas JT, McDonald JM (2005) RhoA and cytoskeletal disruption mediate reduced osteoblastogenesis and enhanced adipogenesis of human mesenchymal stem cells in modeled microgravity. J Bone Miner Res 20(10):1858–1866
Mohebbi-Kalhori D, Behzadmehr A, Doillon CJ, Hadjizadeh A (2012) Computational modeling of adherent cell growth in a hollow-fiber membrane bioreactor for large-scale 3-D bone tissue engineering. J Artif Organs 15(3):250–265
Bettahalli NM, Vicente J, Moroni L, Higuera GA, van Blitterswijk CA, Stamatialis DF, Wessling M (2011) Integration of hollow fiber membranes improves nutrient supply in three-dimensional tissue constructs. Acta Biomater 7(9):3312–3324
Oncül AA, Kalmbach A, Genzel Y, Reichl U, Thévenin D (2010) Characterization of flow conditions in 2 L and 20 L wave bioreactors using computational fluid dynamics. Biotechnol Prog 26(1):101–110
Dubey H, Das SK, Panda T (2006) Numerical simulation of a fully baffled biological reactor: the differential circumferential averaging mixing plane approach. Biotechnol Bioeng 95(4):754–766
Kim JL, Park SH, Jeong D, Nam JS, Kang YH (2012) Osteogenic activity of silymarin through enhancement of alkaline phosphatase and osteocalcin in osteoblasts and tibia-fractured mice. Exp Biol Med (Maywood) 237(4):417–428
Song K, Liu T, Xiangqin Li, Cui Z, Sun X, Ma X (2007) Three-dimensional expansion. In suspension culture of SD rat’s osteoblasts in a rotating wall vessel bioreactor. Biomed Environ Sci 20:91–98
Wiren KM, Hashimoto JG, Semirale AA, Zhang XW (2011) Bone vs. fat: embryonic origin of progenitors determines response to androgen in adipocytes andosteoblasts. Bone 49(4):662–672
Acknowledgments
This work was supported by the Fok Ying Tung Education Foundation (132027), National Science Foundation of China (31370991/31170945), the State Key Laboratory of Fine Chemicals (KF1111), and the Fundamental Research Funds for the Central Universities (DUT14YQ106/DUT15QY47), SRF for ROCS, SEM.
Author information
Authors and Affiliations
Corresponding authors
Additional information
K. Song, X. Yan, Y. Zhang and F. Song are co-first authors.
Rights and permissions
About this article
Cite this article
Song, K., Yan, X., Zhang, Y. et al. Numberical simulation of fluid flow and three-dimensional expansion of tissue engineering seed cells in large scale inside a novel rotating wall hollow fiber membrane bioreactor. Bioprocess Biosyst Eng 38, 1527–1540 (2015). https://doi.org/10.1007/s00449-015-1395-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-015-1395-6