Abstract
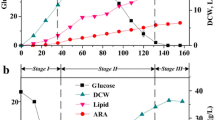
A three-stage fermentation strategy was designed for efficient arachidonic acid (ARA)-rich oil production by Mortierella alpina. The process at different stages by changing the components of medium was investigated. In the first stage, mycelia were inoculated in a nutrient-rich medium for rapid propagation. In the second stage, mycelia were collected and then cultivated in glucose solution to achieve high cellular lipid contents. In the third stage, mycelia were cultured in a glucose-absent medium to obtain rapid ARA accumulation. Using this fermentation strategy, high dry cell weight, lipid, and ARA concentration reached 41.6, 26.6, and 11.4 g/L, respectively. The results demonstrated that mycelia propagation, lipid biosynthesis, and ARA accumulation process can be significantly spatially separated, allowing further optimization to improve the efficiency of each stage. This was the first report of using a three-stage fermentation strategy for ARA-rich oil production, and it could be applied to other similar oleaginous microorganisms to obtain high related polyunsaturated fatty acids accumulation.






Similar content being viewed by others
References
Papanikolaou S, Sarantou S, Komaitis M, Aggelis G (2004) Repression of reserve lipid turnover in Cunninghamella echinulata and Mortierella isabellina cultivated in multiple-limited media. J Appl Microbiol 97:867–875
Certik M, Shimizu S (2000) Kinetic analysis of oil biosynthesis by an arachidonic acid-producing fungus, Mortierella alpina 1S-4. Appl Microbiol Biotechnol 54:224–230
Kendrick A, Ratledge C (1992) Lipids of selected moulds grown for production of n-3 and n-6 polyunsaturated acids. Lipids 27:15–20
Eroshin VK, Satroutdinov AD, Dedyukhina EG, Chistyakova TI (2000) Arachidonic acid production by Mortierella alpina with growth-coupled lipid synthesis. Process Biochem 35:1171–1175
Ji XJ, Ren LJ, Nie ZK, Huang H, Ouyang PK (2013) Fungal arachidonic acid-rich oil: Research, development, and industrialization. Crit Rev Biotechnol. doi:10.3109/07388551.2013.778229
Higashiyama K, Fujikawa S, Park EY, Shimizu S (2002) Production of arachidonic acid by Mortierella fungi. Biotechnol Bioprocess Eng 7:252–262
Singh G, Chandra RK (1988) Biochemical and cellular effects of fish and fish oils. Prog Food Nutr Sci 12:371–419
Gill I, Valivtry R (1997) Polyunsaturated fatty acids, part 1: occurrence, biological activities and applications. Trends Biotechnol 15:401–409
Bajpai PK, Bajpai P, Ward OP (1991) Production of arachidonic acid by Mortierella alpina ATCC 32222. J Ind Microbiol Biotechnol 8:179–186
Sakuradani E, Ando A, Ogawa J, Shimizu S (2009) Improved production of various polyunsaturated fatty acids through filamentous fungus Mortierella alpina breeding. Appl Microbiol Biotechnol 84:1–10
Koike Y, Cai HJ, Higashiyama K, Fujikawa S, Park EY (2001) Effect of consumed carbon to nitrogen ratio on mycelial morphology and arachidonic acid production in cultures of Mortierella alpina. J Biosci Bioeng 91:382–389
Sajbidor J, Dobronova S, Certik M (1990) Arachidonic acid production by Mortierella sp. S-17, influence of C/N ratio. Biotechnol Lett 12:455–456
Folch J, Lees M, Sloane-Stanley G (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
You JY, Peng C, Liu X, Ji XJ, Lu JM, Tong QQ, Wei P, Cong LL, Li ZY, Huang H (2011) Enzymatic hydrolysis and extraction of arachidonic acid rich lipids from Mortierella alpina. Bioresour Technol 102:6088–6094
Zhu M, Yu LJ, Li W, Zhou PP, Li CY (2006) Optimization of arachidonic acid production by fed-batch culture of Mortierella alpina based on dynamic analysis. Enzyme Microb Technol 38:735–740
Papanikolaou S, Aggelis G (2011) Lipids of oleaginous yeasts. Part I: biochemistry of single cell oil production. Eur J Lipid Sci Tech 113:1031–1051
Ratledge C, Wynn J (2002) The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv Appl Microbiol 51:1–51
Wu SG, Hu CM, Jin GJ, Zhao X, Zhao ZK (2010) Phosphate-limitation mediated lipid production by Rhodosporidium toruloides. Bioresour Technol 101:6124–6129
Wu SG, Zhao X, Shen HW, Wang Q, Zhao ZK (2011) Microbial lipid production by Rhodosporidium toruloides under sulfate-limited conditions. Bioresour Technol 102:1803–1807
Papanikolaou S, Komaitis M, Aggelis G (2004) Single cell oil (SCO) production by Mortierella isabellina grown on high-sugar content media. Bioresource Technol 95:287–291
Park EY, Koike Y, Higashiyama K, Fujikawa S, Okabe M (1999) Effect of nitrogen source on mycelial morphology and arachidonic acid production in cultures of Mortierella. J Biosci Bioeng 88:61–67
Kamisaka Y, Noda N, Sakai T, Kawasaki K (1999) Lipid bodies and lipid body formation in an oleaginous fungus, Mortierella ramanniana var. angulispora. Biochim Biophys Acta 1438:185–198
Zhang Y, Goldman S, Baerga R, Zhao Y, Komatsu M, Jin SK (2009) Adipose-specific deletion of autophagy-related gene 7 (atg7) in mice reveals a role in adipogenesis. Proc Natl Acad Sci USA 106:19860–19865
Hamasaki M, Noda T, Baba M, Ohsumi Y (2005) Starvation triggers the delivery of the endoplasmic reticulum to the vacuole via autophagy in yeast. Traffic 6:56–65
Takeshige K, Baba M, Tsuboi S, Noda T, Ohsumi Y (1992) Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J Cell Biol 119:301–311
Eroshin VK, Dedyukhina EG, Satroutdinov AD, Chistyakova TI (2002) Growth-coupled lipid synthesis in Mortierella alpina LPM 301, a producer of arachidonic acid. Microbiol 71:169–172
Acknowledgments
This work was financially supported by the National Science Foundation for Distinguished Young Scholars of China (No. 21225626), the National Basic Research Program of China (No. 2011CBA00807), the National Natural Science Foundation of China (No. 21006049), the National Key Technology Support Program of China (No. 2011BAD23B03), the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT1066), and the Priority Academic Program Development of Jiangsu Higher Education Institutions. H. Huang was supported by the Program for New Century Excellent Talents in University from the Ministry of Education of China (No. NCET-09-0157).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Z.-K. Nie and Z.-T. Deng contributed equally to this work.
Rights and permissions
About this article
Cite this article
Nie, ZK., Deng, ZT., Zhang, AH. et al. Efficient arachidonic acid-rich oil production by Mortierella alpina through a three-stage fermentation strategy. Bioprocess Biosyst Eng 37, 505–511 (2014). https://doi.org/10.1007/s00449-013-1015-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-013-1015-2