Abstract
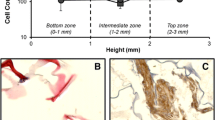
Gelatin-/chitosan-/hyaluronan-based biomaterials are used in tissue engineering as cell scaffolds. Three gamma radiation doses (1, 10 and 25 kGy) were applied to scaffolds for sterilization. Microstructural changes of the irradiated polymers were evaluated by using scanning electron microscopy (SEM) and differential scanning calorimetry (DSC). A dose of 25 kGy produced a rough microstructure with a reduction of the porosity (from 99 to 96 %) and pore size (from 160 to 123 μm). Radiation also modified the glass transition temperature between 31.2 and 42.1 °C (1 and 25 kGy respectively). Human skin cells cultivated on scaffolds irradiated with 10 and 25 kGy proliferated at 48 h and secreted transforming growth factor β3 (TGF-β3). Doses of 0 kGy (non-irradiated) or 1 kGy did not stimulate TGF-β3 secretion or cell proliferation. The specific growth rate and lactate production increased proportionally to radiation dose. The use of an appropriate radiation dose improves the cell scaffold properties of biomaterials.






Similar content being viewed by others
References
Metcalfe AD, Ferguson MWJ (2009) Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interface 4(14):413–437
Griffith LG, Schwartz MA (2006) Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol 7:211–224
Kuntz RM, Saltzman WM (1997) Neutrophil motility in extracellular matrix gels: mesh size and adhesion affect speed of migration. Biophys J 72:1472–1480
Yannas IV, Burke JF (1980) Design of an artificial skin I. Basic design principles. J Biomed Mater Res 14:65–81
Romanelli M, Dini V, Bertone M, Barbanera Sy, Brilli C (2007) OASIS® wound matrix versus Hyaloskin® in the treatment of difficult-to-heal wounds of mixed arterial/venous aetiology. In Wound J 4(1):3–7
Falanga V, Sabolinski ML (1999) A bilayered living skin construct (Apligraf®) accelerates complete closure of hard-to-heal venous ulcers. Wound Repair Regen 7:201–207
Still J, Glat P, Silverstein P, Griswold J, Mozingo D (2003) The use of a collagen sponge/living cell composite material to treat donor sites in burn patients. Burns 29(8):837–841
Zhong SP, Zhang YZ, Lim CT (2010) Tissue scaffolds for skin wound healing and dermal reconstruction. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2(5):510–525
Tan H, Wu J, Lao L, Gao C (2009) Gelatin/chitosan/hyaluronan scaffold integrated with PLGA microspheres for cartilage tissue engineering. Acta Biomater 5:328–337
Weinstein-Oppenheimer CR, Aceituno AR, Brown DI, Acevedo C, Ceriani R, Fuentes MA, Albornoz F, Henriquez-Roldan CF, Morales P, Maclean C, Tapia SM, Young ME (2010) The effect of an autologous cellular gel-matrix integrated implant system on wound healing. J Transl Med 8:59
Liu H, Mao J, Yao K, Yang G, Cui L, Cao Y (2004) A study on a chitosan–gelatin–hyaluronic acid scaffold as artificial skin in vitro and its tissue engineering applications. J Biomater Sci Polym Ed 15:25–40
Huang Y, Onyeri S, Siewe M, Moshfeghian A, Madihally SV (2005) In vitro characterization of chitosan–gelatin scaffolds for tissue engineering. Biomaterials 26:7616–7627
Xia WY, Liu W, Cui L, Liu YC, Zhong W, Liu DL, Wu JJ, Chua KH, Cao YL (2004) Tissue engineering of cartilage with the use of chitosan–gelatin complex scaffolds. J Biomed Mater Res B Appl Biomater 71B:373–380
Plikk P, Odelius K, Hakkarainen M, Albertsson AC (2006) Finalizing the properties of porous scaffolds of aliphatic polyesters through radiation sterilization. Biomater 27:5335–5347
Nanda P, De SK, Manna S, De U, Tarafdar S (2010) Effect of gamma irradiation on a polymer electrolyte: variation in crystallinity, viscosity and ion-conductivity with dose. Nucl Instrum Methods Phys Res B 268:73–78
Sinha M, Goswami MM, Mal D, Middya TR, Tarafdar S, De U, Chaudhuri SK, Das D (2008) Effect of gamma irradiation on the polymer electrolyte PEO-NH4ClO4. Ionics 14:323–327
Parenteau-Bareil R, Gauvin R, Berthod F (2010) Collagen-based biomaterials for tissue engineering applications. Materials 3(3):1863–1887
Siritientong T, Srichana T, Aramwit P (2011) The effect of sterilization methods on the physical properties of silk sericin scaffolds. AAPS Pharm Sci Tech 12:771–781
Acevedo CA, Brown DI, Young ME, Reyes JG (2009) Senescent cultures of human dermal fibroblasts modified phenotype when immobilized in fibrin polymer. J Biomater Sci Polym Ed 20:1929–1942
Enrione J, Osorio F, Lopez D, Weinstein-Oppenheimer C, Fuentes MA, Ceriani R, Brown DI, Albornoz F, Sanchez E, Villalobos P, Somoza RA, Young ME, Acevedo CA (2010) Characterization of a gelatin/chitosan/hyaluronan scaffold-polymer. Electron J Biotechnol 13:15
Acevedo CA, Weinstein-Oppenheimer C, Brown DI, Huebner H, Buchholz R, Young ME (2009) A mathematical model for the design of fibrin microcapsules with skin cells. Bioprocess Biosyst Eng 32:341–351
Gorna K, Gogolewski S (2003) The effect of gamma radiation on molecular stability and mechanical properties of biodegradable polyurethanes for medical applications. Polym Degrad Stab 79:465–474
Goldman M, Pruitt L (1998) Comparison of the effects of gamma radiation and low temperature hydrogen peroxide gas plasma sterilization on the molecular structure, fatigue resistance, and wear behavior of UHMWPE. J Biomed Mater Res 40:378–384
Alijani S, Balaghi S, Mohammadifar MA (2011) Effect of gamma irradiation on rheological properties of polysaccharides exuded by A. fluccosus and A. Gossypinus. Int J Biol Macromol 49:471–479
Rahman MS, Al-Saidi G, Guizani N, Abdullah A (2010) Development of state diagram of bovine gelatin by measuring thermal characteristics using differential scanning calorimetry (DSC) and cooling curve method. Thermochim Acta 509:111–119
Yakimets I, Wellner N, Smith AC, Wilson RH, Farhat I, Mitchell J (2005) Mechanical properties with respect to water content of gelatin films in glassy state. Polymer 46:12585
Sobral PJA, Menegalli FC, Hubinger MD, Roques MA (2001) Mechanical, water vapor barrier and thermal properties of gelatin based edible films. Food Hydrocoll 15:423–432
Fraga AN, Williams RJ (1985) Thermal properties of gelatin films. Polymer 26:113–118
Chin CD, Khanna K, Sia SK (2008) A microfabricated porous collagen-based scaffold as prototype for skin substitutes. Biomed Microdevices 10:459–467
Dziedzic-Goclawska A, Kaminski A, Uhrynowska-Tyszkiewicz I, Michalik J, Stachowicz W (2008) Radiation sterilization of human tissue grafts. In: Trends in radiation sterilization of health care products. International Atomic Energy Agency, Viena
Acevedo CA, Somoza RA, Weinstein-Oppenheimer C, Brown DI, Young ME (2010) Growth factor production from fibrin-encapsulated human keratinocytes. Biotechnol Lett 32:1011–1017
Ferguson MW, O’Kane S (2004) Scar-free healing: from embryonic mechanisms to adult therapeutic intervention. Philos Trans R Soc Lond B Biol Sci 359:839–850
Hao J, Varshney RR, Wang DA (2008) TGF-beta3: a promising growth factor in engineered organogenesis. Expert Opin Biol Ther 8:1485–1493
Schultze-Mosgau S, Wehrhan F, Amann K, Radespiel-Troger M, Rodel F, Grabenbauer GG (2003) In vivo TGF-beta 3 expression during wound healing in irradiated tissue: an experimental study. Strahlenther Onkol 179:410–416
Acknowledgments
The authors wish to thank CONICYT for the Fondecyt Grant (1110607) and FONDEF Grant (D07I1075) and Universidad Técnica Federico Santa María for the Piic2011 fellowship to Rodrigo A. Somoza.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Acevedo, C.A., Somoza, R.A., Weinstein-Oppenheimer, C. et al. Improvement of human skin cell growth by radiation-induced modifications of a Ge/Ch/Ha scaffold. Bioprocess Biosyst Eng 36, 317–324 (2013). https://doi.org/10.1007/s00449-012-0786-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-012-0786-1