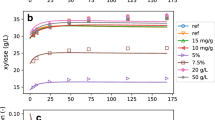
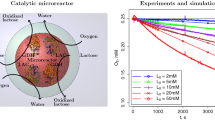
Abstract
A general procedure has been developed to model the behaviour of enzymatic reactions in a membrane bioreactor. This procedure unifies the kinetics of the reaction and the adsorption of the enzyme or enzymatic complexes on the membrane, enabling the selection of the most appropriate kinetic model. The general procedure proposed has been particularized and applied to experimental results obtained with two enzymatic reactions carried out in a hollow-fibre reactor, enzymatic hydrolysis of lactose by β-galactosidase and glucose–fructose isomerization by glucose isomerase. The application of the general model has allowed us to determine the mechanism of the reaction for both kinetic reactions, assuming the adsorption of the enzymatic complex EGa for lactose hydrolysis and the adsorption of the free enzyme onto the membrane for glucose–fructose isomerization.





Similar content being viewed by others
Abbreviations
- C E :
-
Concentration of total active enzyme (g/L)
- E :
-
Concentration of free enzyme present in the reaction medium (M)
- E 1,E 2,...,E e :
-
Concentration of different enzymatic complexes in the medium reaction (M)
- ECS:
-
Extracapillary space
- EGa :
-
Concentration of the enzyme-galactose complex (M)
- EGan :
-
Concentration of the enzyme-galactose complex adsorbed onto the active centre of the membrane (M)
- EL :
-
Concentration of the enzyme-lactose complex (M)
- ELn :
-
Concentration of the enzyme-lactose complex adsorbed onto the active centre of the membrane (M)
- En,E 1 n,E 2 n,...,E e n :
-
Concentration of the enzymatic species E,E 1,E 2,...,E e adsorbed onto the membrane (M)
- e T :
-
Mols of active enzyme per gram of enzyme (mol/g)
- F :
-
Concentration of fructose (M)
- Ga :
-
Concentration of galactose (M)
- Ga 0 :
-
Initial concentration of galactose (M)
- Gl :
-
Concentration of glucose (M)
- Gl 0 :
-
Initial concentration of glucose (M)
- GOD-Perid:
-
Glucose-oxidase peroxidase
- ΔH ad :
-
Adsorption enthalpy (kJ/mol)
- k :
-
Rate constant of Eq. 2 (molglucose mol −1enzyme h−1)
- k 1,k −1,k 1a ,k −1a ,k 2,k −2,k 3,k −3,...,k e ,k −e :
-
Individual kinetic constants of the general model proposed
- K D0,K D1,K D2,...,K De :
-
Equilibrium constants of adsorption of the different enzymatic species onto the membrane (dimensionless)
- K e :
-
Equilibrium constant of glucose–fructose isomerization (dimensionless)
- k g ,k f :
-
Kinetic constants of isomerization (L mol−1 h−1)
- k −g ,k −f :
-
Kinetic constants of isomerization (h−1)
- K I :
-
Equilibrium constant of Eq. 3 (M)
- K M :
-
Michaelis-Menten constant (M)
- K mf :
-
Michaelis-Menten constant for fructose–glucose isomerization (M)
- K mg :
-
Michaelis-Menten constant for glucose–fructose isomerization (M)
- L :
-
Concentration of monohydrate lactose (M)
- L 0 :
-
Initial concentration of monohydrate lactose (M)
- n :
-
Concentration of empty active centres on the membrane surface
- r :
-
Reaction rate of glucose–fructose isomerization (mol g−1 h−1)
- r fru :
-
Reaction rate of fructose–glucose isomerization in the RHFB (mol g−1 h−1)
- RHFB:
-
Recirculation hollow-fibre bioreactor
- r lac :
-
Lactose-hydrolysis reaction rate in a RHFB (mol g−1 h−1)
- S 0 :
-
Initial concentration of substrate in the fructose–glucose isomerization and initial concentration of lactose and galactose in lactose hydrolysis (M)
- t :
-
Time (h)
- T :
-
Temperature
- V mf :
-
Maximum rate reaction in fructose–glucose isomerization (mol g−1 h−1)
- x :
-
Conversion (dimensionless)
- X :
-
Concentration of intermediate complex (M)
- x e :
-
Equilibrium conversion (dimensionless)
- Xn :
-
Concentration of the enzymatic species X adsorbed onto the membrane (M)
- x 0 :
-
Initial conversion in fructose–glucose isomerization (dimensionless)
- y :
-
Treatment intensity (g h/L)
References
Bódalo A, Gómez JL, Gómez E, Máximo MF, Hidalgo AM (2001) Simulation of transient state in enzymatic membrane reactors for resolution of DL-valine and experimental. J Chem Technol Biotechnol 76(9):978–984. DOI:10.1002/jctb.480
Foda MI, Lopez-Leiva M (2000) Continuous production of oligosaccharides from whey using a membrane reactor. Process Biochem 35(6):581–587. DOI:10.1016/S0032-9592(99)00108-9
Nakajima M, Iwasaki K-I, Nabetani H, Watanabe A (1990) Continuous hydrolysis of soluble starch by free β-amylase and pullulanase using an ultrafiltration membrane reactor. Agri Biol Chem 54(11):2793–2799
Sisak C, Nagy E, Burfeind J, Schügerl K (2000) Technical aspects of separation and simultaneous enzymatic reaction in multiphase enzyme membrane reactors. Bioproc Biosyst Eng 23(5):503–512
Kohlwey DE, Cheryan M (1981) Performance of a β-D-galactosidase hollow fiber reactor. Enzyme Microb Technol 3(1):64–68. DOI:10.1016/0141-0229(81)90038-7
Korus RA, Olson AC (1977) The use of α-galactosidase and invertase in hollow fiber reactors. Biotechnol Bioeng 19(1):1–8. DOI:10.1002/bit.260190102
Korus RA, Olson AC (1977) Use of glucose isomerase in hollow fiber reactors. J Food Sci 42(1):258–260
Malcata FX, García HS, Hill CG, Amundson CH (1992) Hydrolysis of butteroil by immobilized lipase using a hollow-fiber reactor: Part I. Lipase adsorption studies. Biotechnol Bioeng 39(6):647–657. DOI:10.1002/bit.260390609
Goto M, Goto M, Nakashio F, Yoshizuka K, Inoue K (1992) Hydrolysis of triolein by lipase in a hollow fiber reactor. J Membr Sci 74(3):207–214. DOI:10.1016/0376-7388(92)80061-N
Tsai S-W, Shaw S-S (1998) Selection of hydrophobic membranes in the lipase-catalyzed hydrolysis of olive oil. J Membr Sci 146(1):1–8. DOI:10.1016/S0376-7388(98)00098-2
Paiva AL, Balcao VM, Malcata FX (2000) Kinetics and mechanisms of reactions catalyzed by immobilized lipases. Enzyme Microb Technol 27(3–5):187–204. DOI:10.1016/S0141-0229(00)00206-4
Balcao VM, Vieira MC, Malcata FX (1996) Adsorption of protein from several commercial lipase preparations onto a hollow-fiber membrane module. Biotechnol Prog 12(2):164–172. DOI:10.1021/bp950070n
Jurado E, Camacho F, Luzón G, Vicaria JM (2000) Recirculation hollow-fiber bioreactor for enzymatic reactions: kinetic model for fructose-glucose isomerization. Afinidad 42(488):283–288
Jurado E, Camacho F, Luzón G, Vicaria JM (2004) Kinetic model for lactose hydrolysis in a recirculation hollow-fiber bioreactor. Chem Eng Sci 59(2):397–405. DOI:10.1016/j.ces.2003.09.035
Aimar P, Baklouti S, Sánchez V (1986) Membrane-solute interactions: influence on pure solvent transfer during ultrafiltration. J Membr Sci 29(2):207–224. DOI:10.1016/S0376-7388(00)82470-9
Werner W, Rey HG, Wielinger H (1970) Properties of a new chromogen for the determination of glucose in blood according to the GOD/POD method. Zeitschrift fur Analytische Chemie Fresenius 252(2–3):224–228
Norde W (2000) Physical chemistry of biological interfaces. Baszkin A, Norde W (eds) Marcel Dekker, New York
Sun S, Yue Y, Huang X, Meng D (2003) Protein adsorption on blood-contact membranes. J Membr Sci 222(1–2):3–18. DOI:10.1016/S0376-7388(03)00313-2
Bódalo A, Gómez JL, Gómez E, Bastida J, Máximo MF, Montiel MC (2001) Ultrafiltration membrane reactors for enzymatic resolution of amino acids: design model and optimization. Enzyme Microb Technol 28(4–5):355–361. DOI:10.1016/S0141-0229(00)00329-X
Carrara CR, Rubiolo AC (1996) Determination of kinetics parameters for free and immobilized β-galactosidase. Proc Biochem 31(3):243–248. DOI:10.1016/0032-9592(95)00056-9
Illanes A, Wilson L, Tomasello G (2001) Effect of modulation of enzyme inactivation on temperature optimization for reactor operation with chitin-immobilized lactase. J Mol Catal B-Enzym 11(4–6):531–540. DOI: 10.1016/S1381-1177(00)00023-0
Jurado E, Camacho F, Luzón G, Vicaria JM (2002) A new kinetic model proposed for enzymatic hydrolysis of lactose by a β-galactosidase from Kluyveromyces fragilis. Enzyme Microb Technol 31(3):300–309. DOI:10.1016/S0141-0229(02)00107-2
Ladero M, Santos A, García-Ochoa F (2000) Kinetic modeling of lactose hydrolysis with an immobilized β-galactosidase from Kluyveromyces Fragilis. Enzyme Microb Technol 27(8):583–592. DOI:10.1016/S0141-0229(00)00244-1
Santos A, Ladero M, García-Ochoa F (1998) Kinetic modeling of lactose hydrolysis by a β-galactosidase from Kluyveromyces Fragilis. Enzyme Microb Technol 22(7):558–567. DOI:10.1016/S0141-0229(97)00236-6
Jurado E, Camacho F, Luzón G, Vicaria JM (2004) Kinetic models of activity for β-galactosidases: influence of pH, ionic concentration and temperature. Enzyme Microb Technol 34(1):33–40. DOI:10.1016/j.enzmictec.2003.07.004
Camacho-Rubio F, Jurado-Alameda E, González-Tello P, Luzón-González G (1996) A comparative study of the activity of free and immobilized enzymes and its application to glucose isomerase. Chem Eng Sci 51(17):4159–4165. DOI:10.1016/0009-2509(96)00249-7
Zhou QZ, Chen XD, Li X (2003) Kinetics of lactose hydrolysis by β-galactosidase of Kluyveromyces lactis immobilized on cotton fabric. Biotechnol Bioeng 81(2):127–133. DOI:10.1002/bit.10414
Cheryan M (1998) Fouling and cleaning. In: Technomic Publishing Company Inc. Ed., Ultrafiltration and Microfiltration Handbook, Lancaster, Pensylvania, p 237
Norde W, MacRitchie F, Nowicka G, Lyklema J (1985) Protein adsorption at solid-liquid interfaces: reversibility and conformation aspects. J Colloid Interf Sci 112(2):447–456. DOI:10.1016/0021-9797(86)90113-X
Jurado-Alameda E, González-Tello P, Luzón-González G (1994) Estudio cinético de la isomerización fructosa-glucosa en un reactor de lecho fijo a 50°C. Afinidad 499:24–30
Acknowledgements
We are grateful to Novozymes for providing the enzyme Lactozym and to Genencor International for providing the enzyme Spezyme GI. We also thank Sorin-Biomédica for providing the hollow-fibre modules used in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jurado, E., Camacho, F., Luzón, G. et al. Kinetic and enzymatic adsorption model in a recirculation hollow-fibre bioreactor. Bioprocess Biosyst Eng 28, 27–36 (2005). https://doi.org/10.1007/s00449-005-0007-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-005-0007-2