Abstract
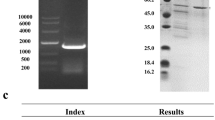
The starch-binding domain of Bacillus sp. strain TS-23 α-amylase was introduced into the C-terminal end of Bacillus kaustophilus leucine aminopeptidase (BkLAP) to generate a chimeric enzyme (BkLAPsbd) with raw-starch-binding activity. BkLAPsbd, with an apparent molecular mass of approximately 65 kDa, was overexpressed in Escherichia coli M15 cells and purified to homogeneity by nickel–chelate chromatography. Native PAGE and chromatographic analyses revealed that the purified fusion protein has a hexameric structure. The half-life for BkLAPsbd was 12 min at 70°C, while less than 20% of wild-type enzyme activity retained at the same heating condition. Compared with the wild-type enzyme, the 60% decrease in the catalytic efficiency of BkLAPsbd was due to a 91% increase in K m value. Starch-binding assays showed that the K d and B max values for the fusion enzyme were 2.3 μM and 0.35 μmol/g, respectively. The adsorption of the crude BkLAPsbd onto raw starch was affected by starch concentration, pH, and temperature. The adsorbed enzyme could be eluted from the adsorbent by 2% soluble starch in 20 mM Tris–HCl buffer (pH 8.0). About 49% of BkLAPsbd in the crude extract was recovered through one adsorption–elution cycle with a purification of 11.4-fold.







Similar content being viewed by others
References
Goldberg AD, Cascio P, Saric T, Rock KL (2002) The importance of the proteasome and subsequent proteolytic steps in the generation of antigenic peptides. Mol Immunol 39:147–164
Saric T, Graef CI, Goldberg AL (2004) Pathway for degradation of peptides generated by proteasomes: a key role for thimet oligopeptidase and other metallopeptidases. J Biol Chem 279:46723–46732
Carpenter FH, Vahl JM (1973) Leucine aminopeptidase (bovine lens): mechanism of activation by Mg2+ and Mn2+ of the zinc metalloenzyme, amino acid composition, and sulfhydryl content. J Biol Chem 248:294–304
Burley SK, David PR, Sweet RM, Taylor A, Lipscomb WN (1992) Structure determination and refinement of bovine lens leucine aminopeptidase and its complex with bestatin. J Mol Biol 224:113–140
Kim H, Lipscomb WN (1993) Differentiation and identification of the two catalytic metal binding sites in bovine lens leucine aminopeptidase by X-ray crystallography. Proc Natl Acad Sci USA 90:5006–5010
Sträter N, Lipscomb WN (1995) Two-metal ion mechanism of bovine lens leucine aminopeptiase: active site solvent structure and binding mode of L-leucinal, a gem-diolate transition state analogue, by X-ray crystallography. Biochemistry 34:14792–14800
Sträter N, Sun L, Kantrowitz ER, Lipscomb WN (1999) A bicarbonate ion as a general base in the mechanism of peptide hydrolysis by zinc leucine aminopeptidase. Proc Natl Acad Sci USA 96:11151–11155
Gu YQ, Walling LL (2002) Identification of residues critical for activity of the wound-induced leucine aminopeptidase (LAP-A) of tomato. Eur J Biochem 269:1630–1640
Chi MC, Chou WM, Hsu WH, Lin LL (2004) Identification of amino acid residues essential for the catalytic reaction of Bacillus kaustophilus leucine aminopeptidase. Biosci Biotechnol Biochem 68:1794–1797
Bayer EA, Shimon LJ, Shoham Y, Lamed R (1998) Cellulosomes: structure and ultrastructure. J Struct Biol 124:221–234
Tsuji SY, Wu N, Khosla C (2001) Intermodular communication in polykeyide synthases: comparing the role of protein-protein interactions to those in other multidomain proteins. Biochemistry 40:2317–2325
Bulow L, Mosbach K (1991) Multienzyme systems obtained by gene fusion. Trends Biotechnol 9:226–231
Nixon AE, Ostermeier M, Benkovic SJ (1998) Hybrid enzymes: manipulating enzyme design. Trends Biotechnol 16:258–264
Beguin P (1999) Hybrid enzymes. Curr Opin Biotechnol 10:336–340
Levy I, Shoseyov O (2002) Cellulose-binding domains: industrial and biotechnological application. Biotechnol Adv 20:191–213
Smith DB, Johnson KS (1988) Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene 67:31–40
Nilsson J, Stahl S, Lundeberg J, Uhen M, Nygren PA (1997) Affinity fusion strategies for detection, purification, and immobilization of recombinant proteins. Protein Expr Purif 11:1–16
Terpe K (2003) Overview of tag protein fusions: from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biotechnol 60:523–533
Greenwood JM, Gilkes NR, Kilburn DG, Miller RJ, Warren RA (1989) Fusion to an endoglucanase allows alkaline phosphatase to bind to cellulose. FEBS Lett 244:127–131
Ramirez C, Fung J, Miller RCJ, Warren RAJ, Kilburn DG (1993) A bifunctional affinity linker to couple antibodies to cellulose. Bio/Technology 11:1570–1573
Shpigel E, Goldlust A, Eshel A, Ber IK, Efroni G, Singer Y, Levy I, Dekel M, Shoseyov O (2000) Expression, purification and applications of staphylococcal protein A fused to cellulose-binding domain. Biotechnol Appl Biochem 31:197–203
Le KD, Gilkes NR, Kilnurn DG, Miller RJ, Saddler JN, Warren RA (1994) A streptavidin-cellulose-binding domain fusion protein that binds biotinylated proteins to cellulose. Enzyme Microb Technol 16:496–500
Svensson B, Jesperson H, Sierks MR, MacGregor EA (1989) Sequence homology between putative raw starch-binding domains from different starch-degrading enzymes. Biochem J 264:309–311
Lawson CL, van Montfort R, Strokopytov B, Rozeboom HJ, Kalk KH, de Vries GE, Penninga D, Dijkhuizen L, Dijkstra BW (1994) Nucleotide sequence and X-ray structure of cyclodextrin glycosyltransferase from Bacillus circulans strain 251 in a maltose-dependent crystal form. J Mol Biol 236:590–600
Sorimachi K, Jacks AJ, Le Gal-Coeffet MF, Williamson G, Archer DB, Williamson MP (1996) Solution structure of the granular starch-binding domain of glucoamylase from Aspergillus niger by nuclear magnetic resonance spectroscopy. J Mol Biol 259:970–987
Janeček Š, Ševčĺk J (1999) The evolution of starch-binding domain. FEBS Lett 456:119–125
Le Gal-Coeffet MF, Jacks AJ, Sorimachi K, Williamson MP, Williamson G, Archer DB (1995) Expression in Aspergillus niger of the starch-binding domain of glucoamylase: comparison with the proteolytically produced starch-binding domain. Eur J Biochem 233:561–567
Wind RD, Butteaar RM, Dijkhuizen L (1998) Engineering of factors determining α-amylase and cyclodextrin glycosyltransferase specificity in the cyclodextrin glycosyltransferase from Thermoanaerobacterium thermosulfurigenes EM1. Eur J Biochem 253:598–605
Lin LL, Lo HF, Chi MC, Ku KL (2003) Functional expression of the raw starch-binding domain of Bacillus sp. strain TS-23 α-amylase in recombinant Escherichia coli. Starch/Stärke 55:197–202
Shibuya T, Tamura G, Shima H, Ishikawa T, Hara S (1992) Construction of an α-amylase/glucoamylase fusion gene and its expression in Saccharomyces cerevisiae. Biosci Biotechnol Biochem 56:884–889
Tsuchiya K, Nagashima T, Yamamoto Y, Gomi K, Kitamoto K, Kumagai C, Tamura G (1994) High level secretion of calf chymosin using a glucoamylase-prochymosin fusion gene in Aspergillus oryzae. Biosci Biotechnol Biochem 58:895–899
Dalmia BK, Schütte K, Nikolov ZL (1995) Domain E of Bacillus macerans cyclodextrin glucanotransferase: an independent starch-binding domain. Biotechnol Bioeng 47:575–584
Ohdan K, Kuriki T, Takata H, Kaneko H, Okada S (2000) Introducing of raw starch-binding domain into Bacillus subtilis α-amylase by fusion with the starch-binding domain of Bacillus cyclomaltodextrin glucanotransferase. Appl Environ Microbiol 66:3058–3064
Hua YW, Chi MC, Lo HF, Hsu WH, Lin LL (2004) Fusion of Bacillus stearothermophilus leucine aminopeptidase II with the raw-starch-binding domain of Bacillus sp. strain TS-23 α-amylase generates a chimeric enzyme with enhanced thermostability and catalytic activity. J Ind Microbiol Biotechnol 31:273–277
Lin LL, Hsu WH, Chu WS (1997) A gene encoding for an α-amylase from thermophilic Bacillus sp. TS-23 and its expression in Escherichia coli. J Appl Microbiol 82:325–334
Lin LL, Hsu WH, Wu CP, Chi MC, Chou WM, Hu HY (2004) A thermostable leucine aminopeptidase from Bacillus kaustophilus CCRC 11223. Extremophiles 8:79–87
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227:680–685
Bradford MM (1976) A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Swillens S (1995) Interpretation of binding curves obtained with high receptor concentrations: practical aid for computer analysis. Mol Pharmacol 47:1197–1203
Hellman J, Mäntsälä P (1992) Construction of an Escherichia coli export-affinity vector for expression and purification of foreign proteins by fusion to cyclomaltodextrin glucanotransferase. J Biotechnol 23:19–34
Moraes LMP, Astolfi-filho S, Oliver SG (1995) Development of yeast strains for the efficient utilization of starch: evaluation of constructs that express α-amylase and glucoamylase separately or as bifunctional fusion proteins. Appl Microbiol Biotechnol 43:1067–1076
Fontes CM, Hazlewood GP, Morag E, Hall J, Hirst BH, Gilbert HJ (1995) Evidence for a general role for non-catalytic thermostabilizing domains in xylanases from thermophilic bacteria. Biochem J 307:151–158
Riedel K, Ritter J, Bauer S, Bronnenmeier K (1998) The modular cellulase CelZ of the thermophilic bacterium Clostridium stercorarium contains a thermostabilizing domain. FEMS Microbiol Lett 164:261–267
Chang HY, Irwin PM, Nikolov ZL (1998) Effects of mutations in the starch-binding domain of Bacillus macerans cyclodextrin glycosyltransferase. J Biotechnol 65:191–202
Lo HF, Lin LL, Chiang WY, Chi MC, Hsu WH, Chang CT (2002) Deletion analysis of the C-terminal region of the α-amylase of Bacillus sp. strain TS-23. Arch Microbiol 178:115–123
Medda S, Saha BC, Ueda S (1982) Raw starch adsorption and elution behavior of glucoamylase I of black Aspergillus. J Ferment Technol 60:261–264
Fang TY, Lin LL, Hsu WH (1994) Recovery of isoamylase from Pseudomonas amyloderamosa by adsorption–elution on raw starch. Enzyme Microb Technol 16:247–252
Saha BC, Ueda S (1983) Raw starch adsorption, elution, and digestion behavior of glucoamylase of Rhizopus niveus. J Ferment Technol 61:67–72
Dalmia BK, Nikolov ZL (1991) Characterization of glucoamylase adsorption to raw starch. Enzyme Microb Technol 13:982–990
Flora K, Brennan JD, Baker GA, Doody MA, Bright FV (1998) Unfolding of acrylodan-labeled human serum albumin probed by steady-state and time-resolved fluorescence methods. Biophys J 75:1084–1096
Giacomelli CE, Norde W (2001) The adsorption-desorption cycle: reversibility of the BSA-silica system. J Colloid Interface Sci 233:234–240
Bourne Y, Henrissat B (2001) Glycoside hydrolases and glycosyltransferases: families and functional modules. Curr Opin Struct Biol 11:593–600
Simpson PJ, Xie H, Bolam DN, Gilbert HJ, Williamson MP (2000) The structural basis for the ligand specificity of family 2 carbohydrate-binding modules. J Biol Chem 275:41137–41142
Leloup VM, Colonna P, Ring SG (1991) α-Amylase adsorption on starch crystallites. Biotechnol Bioeng 38:127–134
Saha BC, Lecureux LW, Zeikus JG (1988) Raw starch adsorption-desorption purification of a thermostable β-amylase from Clostridium thermosulfurogenes. Anal Biochem 175:569–572
Haska N, Ohta Y (1992) Mechanism of hydrolysis of the treated sago starch granules by raw starch digesting amylases from Pencillium brunneum. Starch/Stärke 44:25–28
Sreenath HK (1992) Study on starch granules digestion by α-amylase. Starch/Stärke 44:61–63
Acknowledgements
This work was supported by a research grant (NSC 93-2313-B-415-004) from the National Science Council of the Republic of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, HB., Chi, MC., Hsu, WH. et al. Construction and one-step purification of Bacillus kaustophilus leucine aminopeptidase fused to the starch-binding domain of Bacillus sp. strain TS-23 α-amylase. Bioprocess Biosyst Eng 27, 389–398 (2005). https://doi.org/10.1007/s00449-005-0001-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-005-0001-8