Abstract
Background
Cyclodextrin glucanotransferases (CGTases) catalyze the synthesis of cyclodextrins, cyclic oligosaccharides composed of glucose monomers that find applications in the pharmaceutical, food, and cosmetic industries. An economic application of these industrially important enzymes requires their efficient production and recovery. In this study, the effect of Sec-type signal peptides on the recombinant expression of a CGTase derived from Bacillus sp. G825-6 was investigated in Escherichia coli BL21(DE3) using a codon-adapted gene. In addition, a novel purification method for the CGTase using starch adsorption was developed.
Results
Expression vectors encoding N-terminal PelB, DacD, and the native Bacillus sp. G825-6 CGTase signal peptides (SP) were constructed for the recombinant CGTase. With the DacD SP derived from E. coli, a 3.9- and 3.1-fold increase in total enzyme activity was obtained compared to using the PelB and the native CGTase SP, respectively. DacD enabled a 7.3-fold increase of activity in the extracellular fraction after induction for 24 h compared to the native CGTase SP. After induction for 48 h, 75% of the total activity was detected in the extracellular fraction. By a batch wise adsorption to starch, the extracellular produced CGTase could be purified to homogeneity with a yield of 46.5% and a specific activity of 1637 U/mg.
Conclusions
The signal peptide DacD promoted the high-level heterologous extracellular expression of a recombinant CGTase from Bacillus sp. G825-6 with a pET20b(+) vector in E. coli BL21(DE3). A protocol based on starch adsorption enabled a fast and efficient purification of the recombinant enzyme.
Similar content being viewed by others
Background
Cyclodextrins (CD) are cyclic oligosaccharides derived from starch. They are synthesized industrially by cyclodextrin glucanotransferases (CGTases, EC 2.4.1.19) [1, 2]. The smallest naturally occurring CD produced by CGTases is cyclomaltohexaose (CD6) composed of six glucose monomers [3]. Their property to form reversible complexes with guest molecules has enabled the application of CD6, CD7, and CD8 in many industrial areas where their demand is still growing. Larger CD, which could find interesting novel applications, have not reached the market yet since efficient processes for their production and purification are still not available [4]. CGTases form a range of CD of different ring sizes with CD6–CD8 as major and larger CD as minor products [5]. In addition to the production of CD, CGTases also find applications e.g. as anti-staling agents in the bakery industry or for the glycosylation of biomolecules [6, 7].
Cyclodextrin glucanotransferases are often produced using the original host organism or recombinantly in Escherichia coli, Bacillus subtilis and Bacillus megaterium [8, 9]. In these bacterial expression systems, a codon usage adaption for the respective expression host could efficiently increase the yield of the recombinant CGTase [8,9,10]. In E. coli, recombinant proteins are translocated into the extracellular space only to a limited extent [11, 12]. The recombinant extracellular expression of CGTases in E. coli has however the advantage of a simplified low-cost downstream process [13,14,15,16]. The addition of divalent metal cations, glycine or Triton X100 has been described to promote the extracellular expression of recombinant CGTase in E. coli [15, 17]. A further strategy is the use of signal peptides (SP) to promote the translocation of the recombinant enzyme. The recombinant SP PelB [18], OmpA [14], and SP derived from the host strains [13] have been shown to facilitate the extracellular expression of CGTases in E. coli. The extracellular expression of the Bacillus sp. G1 CGTase with the native G1-CGTase SP in E. coli resulted in a yield of 62.3% of the total activity in the culture medium [13]. A CGTase gene from Paenibacillus macerans JFB05-01 was cloned into the pET20b(+) vector with the SP OmpA [14]. Using a fed-batch protein expression strategy, up to 79% of the total activity was detected in the culture medium.
In this study, we compared the effect of PelB, DacD, and the native Bacillus sp. G825-6 CGTase SP on the extracellular expression of recombinant CGTase in E. coli. In addition, we showed that the extracellular expressed CGTase could be efficiently purified by batch wise adsorption to starch in a single step.
Methods
Construction of expression vectors
The codon-optimized gene coding for the Bacillus sp. G825-6 CGTase (cgtS) was cloned into pET20b(+) as described before [19, 20] and enabled the production of a recombinant CGTase with a N-terminal PelB SP. The gene encoding the DacD SP was synthesized by Life Technologies (Carlsbad, CA, USA) and cloned into the pET20b(+):cgtS vector via the restriction sites NdeI and BamHI. Originally, dacD describes the gene encoding d-alanyl-d-alanine carboxypeptidase of E. coli. In this study, we refer only to the corresponding SP of DacD. The gene encoding for the DacD SP was introduced via the restriction sites NdeI and BamHI to replace the gene for PelB SP in the pET-20b(+) vector. A construct without any SP was prepared by eliminating the pelB leader sequence using the NdeI restriction site.
Recombinant protein expression
Lysogeny broth (LB) medium containing 100 µg/ml ampicillin was used to grow E. coli at 37 °C. Flasks with 250 ml medium were inoculated with overnight cultures to an optical density of 0.1 at 600 nm (OD600). Recombinant protein expression was induced with isopropyl-β-d-thiogalactopyranoside (IPTG) at a final concentration of 0.5 mM at an OD600 of 0.6. After induction, cultures were further incubated for up to 48 h at 25 °C under vigorous shaking.
Cell fractionation
Aliquots (2 ml) were collected at different incubation times and separated into extracellular and cytoplasmic fractions with the PeriPreps™ Periplasting Kit (Epicentre, Madison, WI, USA).
Protein quantification
Protein concentration was measured using the Bradford method with Rotiquant according to the supplier manual (Carl Roth, Karlsruhe, Germany). The assay was performed in a 96-well microplate format with a double determination at 450 and 590 nm [21]. Bovine serum albumin was used for calibration.
Starch-degrading activity assay
One unit of starch-degrading activity was defined as the amount of CGTase that degrades 1 mg starch in 10 min at pH 8.5 and 50 °C [22]. The assay was performed as described previously [23] with the following modifications: starch solution (12 g/l) was prepared with CGTase buffer (25 mM Tris–HCl, 10 mM KCl, 10 mM MgCl2, pH 8.5). Enzyme solution (20 µl) was added to 100 µl of the starch solution at 50 °C.
Purification of the recombinant CGTase by starch adsorption
A soluble starch solution (50 g/l) was prepared in CGTase buffer. The solution was boiled and immediately chilled to 4 °C for 24 h. The starch gel formed was used for the batch wise adsorption of the CGTase. A slurry (15 ml) was prepared by dilution of the starch gel with CGTase buffer (1:2). The mixture was centrifuged, the supernatant was discarded and the extracellular fraction obtained after the expression of the DacD-CGTase (100 ml) was added and mixed. After 20 min at 4 °C, the mixture was centrifuged at 1500×g for 3 min and the supernatant was removed. Two washing steps with CGTase buffer (45 ml) were performed. The CGTase was eluted by adding 45 ml of CGTase buffer containing 0.05 g maltose and 0.6 g KCl to the starch and incubated at 50 °C for 1.5 h. The mixture was centrifuged at 9000×g for 10 min at room temperature. The supernatant was removed and further centrifuged at 15,000×g for 15 min followed by filtration with a 0.22 µm filter. The eluate was concentrated using an ultrafiltration tube with a molecular weight cut-off of 50 kDa (Merck Millipore, Darmstadt, Germany) to a final volume of 1.5 ml.
The purification of the CGTase from the intracellular fractions obtained with the pelB construct was performed with 15 ml crude extract obtained from 1 l of culture. Cells were harvested and resuspended in 10 ml CGTase buffer. Cell disruption was accomplished by five cycles of ultrasonic treatment (Sonoplus HD 2200, tip KE 76, Bandelin Electronic, Germany, Berlin). The lysate was centrifuged at 13,000×g for 30 min at 4 °C and the supernatant was used as crude extract for purification as described above for the extracellular fraction.
CGTase purification by immobilized metal affinity chromatography (IMAC)
A 1 ml HisTrap FF column (GE Healthcare, Freiburg, Germany) was used for the purification of the CGTase with a Äkta Pure chromatography system (GE Healthcare, Freiburg, Germany). Buffer A consisted of 20 mM sodium phosphate buffer, pH 7.4 and 500 mM NaCl and buffer B consisted of buffer A with additional 250 mM of imidazole. Equilibration was performed with 5 column volumes of 96% buffer A and 4% buffer B. Extracellular supernatant (100 ml) was loaded onto the column, followed by a washing step of 2.5 CV of 96% buffer A and 4% buffer B. Elution of the CGTase was performed using a gradient from 4% buffer B to 100% buffer B within five column volumes. Fractions of 0.5 ml were collected.
SDS-PAGE
Protein samples were analyzed using 12% polyacrylamide gels with 4% stacking gel [24]. Pierce Unstained Protein MW Marker was used (Thermo Scientific, Schwerte, Germany). Extracellular fractions were precipitated by 10% (v/v) trichloroacetic acid and concentrated approximately tenfold [25].
Results and discussion
Recombinant expression of the CGTase
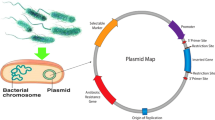
The codon-optimized cgtS gene encoding the Bacillus sp. G825-6 CGTase were expressed with the PelB, DacD, and the native CgtS SP in E. coli BL21(DE3) harboring a pET20b(+) vector (Fig. 1).
Figure 2 shows the growth curves of E. coli BL21(DE3) harboring the four different plasmids and the corresponding starch-degrading activity in the extra- and intracellular fractions of the cultures. The constructs containing the DacD SP produced an approximately 3.9, 3.2, and 2.7-fold higher activity than those containing PelB, CgtS, and the construct without a SP, respectively. DacD also caused a pronounced secretion of the enzymatic activity into the extracellular medium. After 24 h, 65% of the total enzyme activity was found in the extracellular space. With DacD, a 2.7- to 4.6-fold higher amount of extracellular enzyme was obtained than with the other SP investigated. These results support a previous analysis of the membrane permeability in E. coli expressing CGTase which has shown that the recombinant protein accumulated near the inner membrane and blocked the translocation channels [26]. The formation of inclusion bodies is a well-known problem limiting the overexpression of proteins in E. coli [27]. Presumably, an efficient translocation of the recombinant protein from the cytoplasm into the periplasm could reduce the formation of inclusion bodies inside the E. coli cells. The translocation into the periplasm is therefore a key factor for the further undirected transport of the recombinant protein into the culture medium. Furthermore, the secretion of recombinant proteins into the extracellular medium greatly simplifies the downstream process by avoiding the disruption of host cells resulting in the release of large amounts of intracellular proteins.
An extended induction time up to 48 h did not result in a further increase in the cell density of the constructs and only a small change of the extracellular activity was detected after 24 h.
SDS-PAGE analysis of the extracellular proteins confirmed the superior expression performance of the recombinant CGTase using the DacD SP (Fig. 3). These results confirmed DacD as a suitable general secretory (Sec) pathway-dependent SP for the efficient extracellular production of the recombinant CGTase in E. coli BL21(DE3).
SDS-PAGE analysis of proteins in the extracellular fractions of cultures expressing CGTase genes with different SP. Lane 1 CGTase without SP; lane 2 CgtS-CGTase; lane 3 PelB-CGTase; lane 4 DacD-CGTase; lane 5 DacD-CGTase purified by batchwise starch adsorption; lane 6: DacD-CGTase obtained with 100 mM glycine added to the culture medium. M molecular mass marker. 10 µg protein for extracellular fractions and 1 µg of purified protein was loaded. CGTase bands are marked with an asterisk
Comparison of DacD with other CGTase-expressing SP
Previous studies have shown that other Sec-dependent SP including PelB and OmpA promoted the transport of recombinant CGTase into the periplasm [15, 28]. However, PelB in our study did not favor a secretion of the CGTase into the extracellular space. By using the native SP of Bacillus sp. G1 CGTase for the recombinant expression in E. coli, 62.3% of the total activity has been detected in the extracellular medium [13]. In contrast, a yield of only 13.2% of the total activity was found in the extracellular medium using the CgtS SP in this study indicating that the SP for an optimal recombinant production of a specific protein cannot be predicted and requires a screening for suitable candidates. While cleavage site prediction is possible to a certain degree [29], the cleavage site of a distinct protein can change from its natural state when expressed with heterogeneous SP [30]. More precise prediction methods have not been developed yet and available models are lacking a profound understanding of the effects of SP on the translocation process.
Effect of glycine on the extracellular expression of DacD-CGTase
The addition of 100 mM glycine immediately after the induction with IPTG resulted in a strong increase in the protein concentration in the extracellular fraction. SDS-PAGE analysis of the extracellular proteins obtained from cultures treated with glycine confirmed the presence of a larger amount of host proteins and a lower proportion of the CGTase suggesting an enhanced cell permeability or even cell lysis of E. coli and the corresponding release of host proteins in the presence of glycine (Fig. 3) [15, 17].
Purification of the recombinant CGTase by starch adsorption and by IMAC
Soluble starch pretreated to form a gel was used to purify the recombinant DacD-CGTase found in the intra- and extracellular fractions by batch wise adsorption. This low-cost and rapid method has previously been described for the purification of other CGTases [31, 32].
As shown in Table 1, 13.1% of the total activity was recovered from the intracellular fraction with a specific activity of 1505 U/mg. In contrast, 46.5% of the total enzyme activity could be recovered from the extracellular fraction indicating that the large amount of host proteins in the intracellular fraction had a negative influence on the adsorption of the CGTase to the starch gel (Table 1a). After a 55-fold concentration of the eluate, a specific activity of 1636.9 U/mg of the purified DacD-CGTase from the extracellular fraction was obtained (Table 1b). For comparison, the recombinant DacD-CGTase was also purified by IMAC. The CGTase in the intracellular fraction could not be separated using this method suggesting that the C-terminal His6-tag did not properly bind to the Ni-Sepharose 6 matrix. As shown in Table 1c, most of the CGTase did also not bind to the column in the absence of E. coli host proteins. Since several intracellular proteins from E. coli have shown a high affinity to Ni2+, further purification steps, e.g. size exclusion chromatography, would be necessary to obtain a homogenous recombinant protein preparation [33]. From the extracellular fractions, only a low yield of CGTase (2.9%) with a specific activity of 595.6 U/mg could be obtained by IMAC (Table 1c). In contrast, by batch wise starch adsorption, highly pure CGTase preparations could be obtained quickly in high yields without the use of expensive IMAC media.
Conclusions
The DacD SP strongly promoted the extracellular production of CGTase in E. coli and enabled an up to 3.3-fold increase in the yield of total activity compared to the other SP. Furthermore, DacD SP caused a significantly enhanced secretion with a more than 13-fold higher specific activity in the extracellular fraction than in the crude cell extract. The extracellular CGTase could be rapidly purified by batch wise starch adsorption with high specific activity and yield. By using these methods, multiple samples can be purified simultaneously and the purification protocol can easily be upscaled to allow for the screening of libraries of CGTase variants obtained by random mutagenesis experiments. A high-level expression system for the extracellular production of CGTases facilitates the manufacture of industrially relevant CD at lower costs opening up new markets for these inclusion complex-forming oligosaccharides.
References
van der Veen BA, van Alebeek GJ, Uitdehaag JC, Dijkstra BW, Dijkhuizen L. The three transglycosylation reactions catalyzed by cyclodextrin glycosyltransferase from Bacillus circulans (strain 251) proceed via different kinetic mechanisms. Eur J Biochem. 2000;267:658–65.
Leemhuis H, Kelly RM, Dijkhuizen L. Engineering of cyclodextrin glucanotransferases and the impact for biotechnological applications. Appl Microbiol Biotechnol. 2010;85:823–35.
Nakagawa T, Ueno K, Kashiwa M, Watanabe J. The stereoselective synthesis of cyclomaltopentaose. A novel cyclodextrin homologue with D.P. five. Tetrahedron Lett. 1994;35:1921–4.
Taira H, Nagase H, Endo T, Ueda H. Isolation, purification and characterization of large-ring cyclodextrins (CD36∼ ∼CD39). J Incl Phenom Macrocycl Chem. 2006;56:23–8.
Qi Q, Zimmermann W. Cyclodextrin glucanotransferase: from gene to applications. Appl Microbiol Biotechnol. 2005;66:475–85.
Shim J, Seo N, Roh S, Kim J, Cha H, Park K. Improved bread-baking process using Saccharomyces cerevisiae displayed with engineered cyclodextrin glucanotransferase. J Agric Food Chem. 2007;55:4735–40.
Zhang Z, Li J, Liu L, Sun J, Hua Z, Du G, et al. Enzymatic transformation of 2-O-α-d-glucopyranosyl-l-ascorbic acid (AA-2G) by immobilized α-cyclodextrin glucanotransferase from recombinant Escherichia coli. J Mol Catal B Enzym. 2011;68:223–9.
Zhou J, Liu H, Du G, Li J, Chen J. Production of α-cyclodextrin glycosyltransferase in Bacillus megaterium MS941 by systematic codon usage optimization. J Agric Food Chem. 2012;60:10285–92.
Jeang C, Lin D, Hsieh S. Characterization of cyclodextrin glycosyltransferase of the same gene expressed from Bacillus macerans, Bacillus subtilis, and Escherichia coli. J Agric Food Chem. 2005;53:6301–4.
Liu H, Li J, Du G, Zhou J, Chen J. Enhanced production of α-cyclodextrin glycosyltransferase in Escherichia coli by systematic codon usage optimization. J Ind Microbiol Biotechnol. 2012;39:1841–9.
Sandkvist M, Bagdasarian M. Secretion of recombinant proteins by Gram-negative bacteria. Curr Opin Biotechnol. 1996;7:505–11.
Mergulhao FJM, Summers DK, Monteiro GA. Recombinant protein secretion in Escherichia coli. Biotechnol Adv. 2005;23:177–202.
Ong RM, Goh KM, Mahadi NM, Hassan O, Rahman RNZRA, Illias RM. Cloning, extracellular expression and characterization of a predominant β-CGTase from Bacillus sp. G1 in E. coli. J Ind Microbiol Biotechnol. 2008;35:1705–14.
Cheng J, Wu D, Chen S, Chen J, Wu J. High-level extracellular production of α-cyclodextrin glycosyltransferase with recombinant Escherichia coli BL21 (DE3). J Agric Food Chem. 2011;59:3797–802.
Ding R, Li Z, Chen S, Wu D, Wu J, Chen J. Enhanced secretion of recombinant α-cyclodextrin glucosyltransferase from E. coli by medium additives. Process Biochem. 2010;45:880–6.
Jonet MA, Mahadi NM, Murad AMA, Rabu A, Bakar FDA, Rahim RA, et al. Optimization of a heterologous signal peptide by site-directed mutagenesis for improved secretion of recombinant proteins in Escherichia coli. J Mol Microbiol Biotechnol. 2012;22:48–58.
Li Z, Li B, Liu Z, Wang M, Gu Z, Du G, et al. Calcium leads to further increase in glycine-enhanced extracellular secretion of recombinant α-cyclodextrin glycosyltransferase in Escherichia coli. J Agric Food Chem. 2009;57:6231–7.
Lee K, Shin H-D, Lee Y-H. Extracellular overproduction of gamma cyclodextrin glucanotransferase in a recombinant E.coli using secretive expression system. J Mol Microbiol Biotechnol. 2002;12:753–9.
Hirano K, Ishihara T, Ogasawara S, Maeda H, Abe K, Nakajima T, et al. Molecular cloning and characterization of a novel γ-CGTase from alkalophilic Bacillus sp. Appl Microbiol Biotechnol. 2006;70:193–201.
Melzer S, Sonnendecker C, Föllner C, Zimmermann W. Stepwise error-prone PCR and DNA shuffling changed the pH activity range and product specificity of the cyclodextrin glucanotransferase from an alkaliphilic Bacillus sp. FEBS Open Bio. 2015;5:528–34.
Zor T, Selinger Z. Linearization of the Bradford protein assay increases its sensitivity: theoretical and experimental studies. Anal Biochem. 1996;236:302–8.
Xiao Z, Storms R, Tsang A. A quantitative starch–iodine method for measuring alpha-amylase and glucoamylase activities. Anal Biochem. 2006;351:146–8.
Qi Q, She X, Endo T, Zimmermann W. Effect of the reaction temperature on the transglycosylation reactions catalyzed by the cyclodextrin glucanotransferase from Bacillus macerans for the synthesis of large-ring cyclodextrins. Tetrahedron. 2004;60:799–806.
Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5.
Bonifacino JS. Metabolic labeling with amino acids. Curr Protoc Cell Biol. 2001;7:7.1.1–1.1026.
Li Z, Su L, Wang L, Liu Z, Gu Z, Chen J, et al. Novel insight into the secretory expression of recombinant enzymes in Escherichia coli. Process Biochem. 2014;49:599–603.
Sørensen HP, Mortensen KK. Advanced genetic strategies for recombinant protein expression in Escherichia coli. J Biotechnol. 2005;115:113–28.
Li B, Wang L, Su L, Chen S, Li Z, Chen J, et al. Glycine and Triton X-100 enhanced secretion of recombinant α-CGTase mediated by OmpA signal peptide in Escherichia coli. Biotechnol Bioprocess Eng. 2012;17:1128–34.
Zhang Z, Henzel WJ. Signal peptide prediction based on analysis of experimentally verified cleavage sites. Protein Sci. 2004;13:2819–24.
Pechsrichuang P, Songsiriritthigul C, Haltrich D, Roytrakul S, Namvijtr P, Bonaparte N, et al. OmpA signal peptide leads to heterogenous secretion of B. subtilis chitosanase enzyme from E. coli expression system. Springerplus. 2016;5:1200.
Pongsawadi P, Yagisawa M. Purification and some properties of cyclomaltodextrin glucanotransferase from Bacillus circulans. Agric Biol Chem. 1988;52:1099–103.
Martins RF, Hatti-Kaul R. A new cyclodextrin glycosyltransferase from an alkaliphilic Bacillus agaradhaerens isolate: purification and characterisation. Enzyme Microb Technol. 2002;30:116–24.
Robichon C, Luo J, Causey TB, Benner JS, Samuelson JC. Engineering Escherichia coli BL21(DE3) derivative strains to minimize E. coli protein contamination after purification by immobilized metal affinity chromatography. Appl Environ Microbiol. 2011;77:4634–46.
Authors’ contributions
CS, RW, TO, WZ conceived the project; CS, RW, TO planned the experiments; EK, JW, CS performed the experiments; EK, CS, JW analysed the data; CS, RW, WZ wrote the paper. All authors read and approved the final manuscript.
Acknowledgements
Support from the German Research Foundation (DFG) and Leipzig University within the program of Open Access Publishing is acknowledged.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding authors on reasonable request.
Funding
CS was supported by the European Social Fund (Project nr. 100234741).
The funding body did not take part in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Sonnendecker, C., Wei, R., Kurze, E. et al. Efficient extracellular recombinant production and purification of a Bacillus cyclodextrin glucanotransferase in Escherichia coli . Microb Cell Fact 16, 87 (2017). https://doi.org/10.1186/s12934-017-0701-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12934-017-0701-1