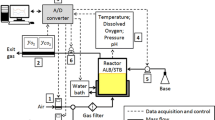
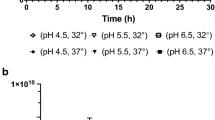
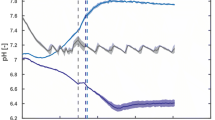
Abstract
High amounts of outer membrane (OM) components were released in glucose-limited fed-batch (GLFB) cultures at 37 °C at specific growth rates approaching 0.05 h−1. Endotoxin analyses from a 20 °C GLFB culture gave similar results. An alternative fermentation technique, the temperature-limited fed-batch (TLFB) technique, reduced the endotoxin concentration in a culture with a biomass concentration of 30 g l−1 from the 850 mg l−1 in traditional GLFB cultures to about 20 mg l−1. The TLFB technique uses the temperature to regulate the dissolved oxygen tension, while all substrate components are unregulated. It appears to be severe glucose limitation that triggers the extensive release of endotoxins rather than a low growth rate. Furthermore, it is not the low temperature that stabilizes the OM when using the TLFB technique. Simulations and experimental data show that this technique results in the same biomass productivity as the GLFB technique.







Similar content being viewed by others
Abbreviations
- LPS:
-
Lipopolysaccharide
- OM:
-
Outer membrane
- GLFB:
-
Glucose limited fed-batch
- TLFB:
-
Temperature limited fed-batch
- EU:
-
Endotoxin unit
- DOT:
-
Dissolved oxygen tension
- OD:
-
Optical density
References
Johnston CJ, Finkelstein JN, Gelein R, Oberdorster G (1998) Pulmonary cytokine and chemokine mRNA levels after inhalation of lipopolysaccharide in C57BL/6 mice. Toxicol Sci 46:300–307
Raetz C (1996) Bacterial lipopolysaccharides: a remarkable family of bioactive macroamphipiles. In: Neidhart FC (ed) Escherichia coli and Salmonella cellular and molecular biology. American Society for Microbiology, Washington, pp 1035–1063
Petsch D, Anspach FB (2000) Endotoxin removal from protein solutions. J Biotechnol 76:97–119
Han L, Enfors S-O, Häggström L (2003) Escherichia coli high-cell-density culture: carbon mass balances and release of outer membrane components. Bioprocess Biosyst Eng 25:205–212
Hoekstra D, van der Laan JW, de Leij L, Witholt B (1976) Release of outer membrane fragments from normally growing Escherichia coli. Biochim Biophys Acta 455:889–899
Marvin HJ, ter Beest MB, Witholt B (1989) Release of outer membrane fragments from wild-type Escherichia coli and from several E. coli lipopolysaccharide mutants by EDTA and heat shock treatments. J Bacteriol 171:5262–5267
Ishiguro EE, Vanderwel D, Kusser W (1986) Control of lipopolysaccharide biosynthesis and release by Esherichia coli and Salmonella typhimurium. J Bacteriol 168:328–333
Bucklin SE, Fujihara Y, Leeson MC, Morrison DC (1994) Differential antibiotic-induced release of endotoxin from gram-negative bacteria. Eur J Clin Microbiol Infect Dis 13:43–51
Loeb MR, Kilner J (1978) Release of a special fraction of the outer membrane from both growing and phage T4-infected Escherichia coli B. Biochim Biophys Acta 518:117–127
Mackowiak PA (1984) Relationship between growth temperature and shedding of lipopolysaccharides by gram-negative Bacilli. Eur J Clin Microbiol 3:406–410
Pelltier C, Bourlioux P, van Heijenoort J (1994) Effects of sub-minimal inhibitory concentrations of EDTA on growth of Escherichia coli and the release of lipopolysaccharide. FEMS Microbiol Lett 117:203–206
Irvin RT, MacAlister TJ, Chan R, Costerton JW (1981) Citrate-tris(hydroxymethyl)aminomethane-mediated release of outer membrane sections from the cell envelope of a deep-rough (heptose defiecient lipopolysaccharide) strain of Escherichia coli O8. J Bacteriol 145:1386–1396
Tsuchido T, Katsui N, Takeuchi A, Takano M, Shibasaki I (1985) Destruction of the outer membrane permeability barrier of Escherichia coli by heat treatment. Appl Environ Microbiol 50:298–303
Zhou L, Srisatjaluk R, Justus DE, Doyle RJ (1998) On the origin of membrane vesicles in gram-negative bacteria. FEMS Microbiol Lett 163:223–228
Wensink J, Witholt B (1981) Outer-membrane vesicles released by normally growing Escherichia coli contain very little lipoprotein. Eur J Biochem 116:331–335
Holme T, Arvidsson S, Lindholm B, Pavlu B (1970) Enzymes: laboratory-scale production. Process Biochem 5:62–66
Silfversparre G, Enfors S-O, Han L, Häggström L, Skogman H (2002) Method for growth of microorganisms, minimising the release of endotoxins from the bacteria to the surrounding medium, Patent (PCT/SE01/02370), International Publication Number WO02/36746A1
Larsson G, Törnkvist M (1996) Rapid sampling, cell inactivation and evaluation of low extracellular glucose concentrations during fed-batch cultivations. J Biotechnol 49:69–82
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Moe D, Garbarsch C, Kirkeby S (1994) The protein effect on determination of DNA with Hoechst 33258. J Biochem Bioph Meth 28:263–276
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Xu B, Jahic M, Enfors S-O (1999) Modeling of overflow metabolism in batch and fed-batch cultures of Escherichia coli. Biotechnol Prog 15:81–90
Rothfield L, Pearlman-Kothencz M (1969) Synthesis and assembly of bacterial membrane components: a lipopolysaccharide-phospholipid-protein complex excreted by living bacteria. J Mol Biol 44:477–492
Prins JM, van Deventer SJH, Kuijper EJ, Speelman P (1994) Clinical relevance of antibiotic-induced endotoxin release. Antimicrob Agents Ch 38:1211–1218
Neidhardt FC, Umbarger EH (1996) Chemical composition of Escherichia coli. In: Neidhart FC (ed) Escherichia coli and Salmonella cellular and molecular biology. American Society for Microbiology, Washington, pp 13–16
Andersson L, Strandberg L, Enfors S-O (1996) Cell segregation and lysis have profound effects on the growth of Escherichia coli in high cell density fed-batch cultures. Biotechnol Prog 12:190–195
Andersson L, Yang S, Neubauer P, Enfors S-O (1996) Impact of plasmid presence and induction on cellular responses in fed-batch cultures of Escherichia coli. J Biotechnol 46:255–263
Cashel M, Gentry DR, Hernandez VJ, Vinella D (1996) The stringent response. In: Neidhart FC (ed) Escherichia coli and Salmonella cellular and molecular biology. American Society for Microbiology, Washington, pp 1458–1496
Teich A, Meyer S, Lin HY, Andersson L, Enfors S-O, Neubauer P (1999) Growth rate related concentration changes of the starvation respons regulators σs and ppGpp in glucose limited fed-batch and continous cultures of Escherichia coli. Biotechnol Prog 15:123–129
Chesbro W (1988) The domains of slow bacterial growth. Can J Microbiol 34(4):427–435
Chalmers JJ, Kim E, Telford JN, Wong EY, Tacon WC, Shuler ML, Wilson DB (1990) Effects of temperature on Escherichia coli overproducing-lactamase or human epidermal growth factor. Appl Environ Microbiol 56:104–111
Jahic M, Wållberg F, Bollok M, Garcia P, Enfors S-O (2003) Temperature limited fed-batch technique for control of proteolysis in Pichia pastoris bioreactor cultures. Microb Cell Fact 2 (see http://www.microbialcellfactories.com/content/2/1/6)
Acknowledgement
This work was supported by a grant from the Swedish Centre for Bioprocess Tecnology, CBioPT.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Svensson, M., Han, L., Silfversparre, G. et al. Control of endotoxin release in Escherichia coli fed-batch cultures. Bioprocess Biosyst Eng 27, 91–97 (2005). https://doi.org/10.1007/s00449-004-0377-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-004-0377-x