Abstract
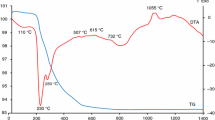
Colors of plinian pumices were measured by spectrocolorimetry, and their quantitative color parameters in the L*a*b* color space were determined. A series of heating experiments of obsidian was conducted to simulate the color-change processes of rhyolitic glasses. In these experiments, following three stages of color-change processes were observed. Stage I showed a rapid b* (yellowishness) increase associated with fast dehydration controlled by water diffusivity (D water). In stage II, a* (reddishness) increase was accompanied by Fe2+ decrease. Both a* increase and Fe2+ decrease can be simulated by a diffusion model. Obtained diffusivity D oxidation were about two orders of magnitude smaller than D water . The a*-value increase after the oxidation in stage III appeared to be quasi-linear with time, indicating the zeroth order reaction corresponding to the formation of hematite-like structures in rhyolitic glasses. The diffusion-limited a* increase model in stage II was applied to a natural plinian pumice fall unit to evaluate time periods of color-change processes through oxidation by air of fragmented rhyolitic materials.









Similar content being viewed by others
References
Baker DR (1992) Tracer diffusion of network formers and multicomponent diffusion in dacitic and rhyolitic melts. Geochim Cosmochim Acta 56:617–631
Burns RG (1993) Mineralogical applications of crystal field theory. Cambridge Topics in Mineral Physics and Chemistry 5. Cambridge University Press, Cambridge, p 551
Cas RAF, Wright JV (1987) Volcanic successions: modern and ancient. Allen & Unwin, London, p 528
Cashman KV, Mangan MT (1994) Physical aspects of magmatic degassing II. Constraints on vesiculation process from textural studies of eruptive products. Rev Mineral 30:447–478
Cook GB, Cooper RF (2000) Iron concentration and physical processes of dynamic oxidation in alkaline earth aluminosilicate glass. Am Mineral 85:397–406
Cooper RF, Fanselow JB, Poker DB (1996) The mechanism of oxidation of a basaltic glass: chemical diffusion of network-modifying cations. Geochim Cosmochim Acta 60:3253–3265
Crank J (1975) The mathematics of diffusion. Oxford Science, Oxford, pp 1–103
Dufek J, Bergantz GW (2005) Transient two-dimensional dynamics in the upper conduit of a rhyolitic eruption: A comparison of closure models for the granular stress. J Volcanol Geotherm Res 143:113–132
Fritz SF, Popp RK (1985) A single-dissolution technique for determining FeO and Fe2O3 in rock and mineral samples. Am Mineral 70:961–968
Gaillard F, Scaillet B, Pichavant M (2002) Kinetics of iron oxidation-reduction in hydrous silisic melts. Am Mineral 87:829–837
Hammer JE, Cashman KV, Hoblitt RP, Newman S (1999) Degassing and microlite crystallization during pre-climatic events of the 1991 eruption of Mt. Pinatubo, Philippines. Bull Volcanol 60:355–380
Keppler H (1992) Crystal field spectra and geochemistry of transition metal ions in silicate melts and glasses. Am Mineral 77:62–75
Klug C, Cashman (1994) Vesiculation of May 18, 1980, Mount St. Helens magma. Geology 22:468–472
Mastin LG (2007) A user-friendly one-dimensional model for wet volcanic plumes. Geochim Geophys Geosyst 8:Q03014 DOI 10.1029/2006GC001455
Mastin LG, Ghiorso MS (2000) A numerical program for steady-state flow of magma-gas mixtures through vertical eruptive conduit. USGS Open-File Report 00-209. USGS, Reston
Miyagi I, Matsubaya O, Nakashima S (1998) Change in D/H ratio, water content and color during dehydration of hornblende. Geochem J 32:33–48
Moriizumi M (1998a) The growth history of the Kuttara Volcanic Group, Hokkaido, Japan. Bull Volcanol Soc Japan 43:95–111 (in Japanese)
Moriizumi M (1998b) The growth history of a caldera volcano—eruptive history and magma plumbing system in the Kuttara volcanic group, Doctoral Thesis. Hokkaido University, Sapporo
Nagano T, Nakashima S (1989) Study of colors and degree of weathering of granitic rocks by visible diffuse reflectance spectroscopy. Geochem J 23:75–83
Nagano T, Nakashima S, Nakayama S, Osada S, Senoo M (1992) Color variations associated with rapid formation of goethite from proto-ferrihydrite at pH13 and 40°C. Clay Clay Mineral 40:600–607
Nagano T, Nakashima S, Nakayama S, Senoo M (1994) The use of color to quantify the effect of pH and temperature on the crystallization kinetics of goethite under highly alkaline conditions. Clay Clay Mineral 42:226–234
Nagano T, Isobe H, Nakashima S, Ashizaki M (2002) Characterization of iron hydroxide in a weathered rock surface by visible micro-spectroscopy. Appl Spectrosc 56:651–657
Nakashima S, Miyagi I, Nakata E, Sasaki H, Nittono S, Hirano T, Sato T, Hayashi H (1992) Color measurement of some natural and synthetic minerals-I. Rep Res Inst Natural Resources Mining College Akita Univ 57:57–76
Nowak M, Keppler H (1998) The influence of water on the environment of transition metals in silicate melts. Am Mineral 83:43–50
Okumura S, Nakashima S (2004) Water diffusivity in rhyolitic glasses as determined by in situ IR spectroscopy. Phys Chem Mineral 31:183–189
Okumura S, Nakamura N, Nakashima S (2003) Determination of molar absorptivity of IR fundamental OH-stretching vibration in rhyolitic glasses. Am Mineral 88:1657–1662
Papale P (2001) Dynamics of magma flow in volcanic conduits with variable fragmentation efficiency and nonequilibrium pumice degassing. J Geophys Res 106:11043–11065
Rossman GR (1988) Spectroscopic methods in mineralogy and geology. Rev Mineral 18:207–254
Schmalzried H (1983) Internal and external oxidation of nonmetallic compounds and solid solutions (I). Ber Bunsenges Phys Chem 87:551–558
Sherman DM, Waite TD (1985) Electronic spectra of Fe3+ oxides and oxide hydroxides in the near IR to near UV. Am. Mineral 70:1262–1269
Stolper EM (1982) Water in silicate glasses: An infrared spectroscopic study. Contrib Mineral Petrol 81:1–17
Spencer KJ, Lindsley DH (1981) A solution model for coexisting iron-titanium oxide. Am Mineral 66:1189–1201
Skogby H, Rossman GR (1989) OH- in pyroxene: an experimental study of incorporation mechanisms and stability. Am Mineral 74:1059–1069
Tait S, Thomas R, Gardner J, Jaupart C (1998) Constraints on cooling rates and permeabilities of pumice in an explosive eruption jet from color and magnetic mineralogy. J Volcanol Geotherm Res 86:79–91
Watson EB (1994) Diffusion in volatile-bearing magmas. Rev Mineral 30:371–412
Woods AW (1988) The fluid dynamics and thermodynamics of eruption columns. Bull Volcanol 50:169–193
Wyszecki Gunter, Stiles WS, Wyszecki Gn (1982) Color science: Concepts and methods, quantitative data and formulae. Wiley, Chichester, pp 117–248
Xu Z, Zhang Y (2002) Quench rate in air, water, and liquid nitrogen, and inference of temperature in volcanic eruption columns. Earth Planet Sci Let 200:315–330
Yamanoi Y, Nakashima S (2005) In-situ high temperature visible microspectroscopy for volcanic materials. Appl Spectrosc 59:1415–1419
Yamanoi Y, Nakashima S, Okumura S, Takeuchi S (2004) Color change measurement of a scoria and simulation heating experiments by spectro-colorimetry. Bull Volcanol Soc Jpn 49:317–331 (in Japanese)
Yamanoi Y, Saiki K, Nakashima S (2007) Color change of basaltic scoria powders under controlled oxygen fugacity conditions. Abstract of Japan Geoscience Union Meeting 2007. V157-P001
Yamanoi Y, Takeuchi S, Okumura S, Nakashima S, Yokoyama T (2008) Color measurements of volcanic ash deposits from three different styles of summit activity at Sakurajima volcano, Japan: conduit processes recorded in color of volcanic ash. J Volcanol Geotherm Res (in press)
Yokoyama T, Nakashima S (2005) Color development of iron oxides during rhyolite weathering over 52,000 years. Chem Geol 219:309–320
Acknowledgements
We thank Dr. N. Furukawa (Chiba University) for his technical supports to determine ferrous iron contents by the phenanthroline method. We are grateful to Ms. Tomitaka, Mr. Masago and Ms. Shiratori for the UV-VIS-NIR spectra measurement at JASCO Co. Ltd. The critical reviews of Drs. H. Shinohara, S. Tait, and I. Miyagi for earlier versions greatly improved the manuscript. We are grateful to two anonymous reviewers for their constructive comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: DB Dingwell
Appendix
Appendix
Color is caused by the reflectance of light in the visible range of the electromagnetic spectrum, i.e., approximately between 400 and 700 nm. The spectral reflectance data obtained by these instruments are converted to three parameters (“tristimulus values” X, Y, and Z) that define the color perceived by the human eye. The tristimulus values can, then, be mathematically converted to color parameters in uniform color spaces, one of them being the CIE-L*a*b* system with the Cartesian coordinates L* (lightness), a*(reddishness–greenishness), and b*(yellowishness–bluishness). Figure 10 represents this L*a*b* color space with their color attributes.
The colors of materials can be measured in the laboratory or on the field by using spectrocolorimeters. Modern commercially available spectrocolorimeters (e.g., Minolta CM2600-d; Fig. 11a) allow a quick measurement of visible reflectance spectra and quantitative color values such as L*, a* and b* of materials. The spectrocolorimeter uses a diffuse illumination from a pulsed xenon light source by using an integrating sphere whose internal surfaces are coated with a white material such as barium sulfate so that the light is uniformly diffused (Fig. 11b). Another light source is also used to measure the reflected light with the same incident angle as the reflected angle to the detector for the specular component. The technical details are given in (http://www.konicaminolta.com/instruments/knowledge/color/).
Rights and permissions
About this article
Cite this article
Moriizumi, M., Nakashima, S., Okumura, S. et al. Color-change processes of a plinian pumice and experimental constraints of color-change kinetics in air of an obsidian. Bull Volcanol 71, 1–13 (2009). https://doi.org/10.1007/s00445-008-0202-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00445-008-0202-5