Abstract
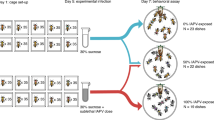
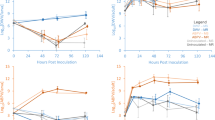
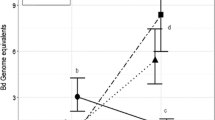
Host–parasite interactions do not occur in a vacuum, but in connected multi-parasite networks that can result in co-exposures and coinfections of individual hosts. These can affect host health and disease ecology, including disease outbreaks. However, many host–parasite studies examine pairwise interactions, meaning we still lack a general understanding of the influence of co-exposures and coinfections. Using the bumble bee Bombus impatiens, we study the effects of larval exposure to a microsporidian Nosema bombi, implicated in bumble bee declines, and adult exposure to Israeli Acute Paralysis Virus (IAPV), an emerging infectious disease from honey bee parasite spillover. We hypothesize that infection outcomes will be modified by co-exposure or coinfection. Nosema bombi is a potentially severe, larval-infecting parasite, and we predict that prior exposure will result in decreased host resistance to adult IAPV infection. We predict double parasite exposure will also reduce host tolerance of infection, as measured by host survival. Although our larval Nosema exposure mostly did not result in viable infections, it partially reduced resistance to adult IAPV infection. Nosema exposure also negatively affected survival, potentially due to a cost of immunity in resisting the exposure. There was a significant negative effect of IAPV exposure on survivorship, but prior Nosema exposure did not alter this survival outcome, suggesting increased tolerance given the higher IAPV infections in the bees previously exposed to Nosema. These results again demonstrate that infection outcomes can be non-independent when multiple parasites are present, even when exposure to one parasite does not result in a substantial infection.



Similar content being viewed by others
Availability of data and material
Data and analysis scripts are available at https://doi.org/10.5061/dryad.3xsj3txk1.
References
Adelman JS, Hawley DM (2017) Tolerance of infection: a role for animal behavior, potential immune mechanisms, and consequences for parasite transmission. Horm Behav 88:79–86
Alger SA et al (2019) RNA virus spillover from managed honeybees (Apis mellifera) to wild bumblebees (Bombus spp.). PLoS One 14(6):e0217822
Alizon S (2013a) Co-infection and super-infection models in evolutionary epidemiology. Interface Focus 3:20130031
Alizon S (2013b) Parasite co-transmission and the evolutionary epidemiology of virulence. Evolution 67:921–933
Alizon S, Van Baalen M (2008) Multiple infections, immune dynamics, and the evolution of virulence. Am Nat 172:150–168
Alizon S et al (2013) Multiple infections and the evolution of virulence. Ecol Lett 16:556–567
Altizer S et al (2004) Seasonal dynamics of mycoplasmal conjunctivitis in eastern North American house finches. J Anim Ecol 73:309–322
Antúnez K et al (2009) Immune suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia). Environ Microbiol 11:2284–2290
Bates D et al (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Baucom RS, de Roode JC (2011) Ecological immunology and tolerance in plants and animals. Funct Ecol 25:18–28
Ben-Ami F et al (2011) The expression of virulence during double infections by different parasites with conflicting host exploitation and transmission strategies. J Evol Biol 24:1307–1316
Blaker EA et al (2014) PCR reveals high prevalence of non/low sporulating Nosema bombi (microsporidia) infections in bumble bees (Bombus) in Northern Arizona. J Invert Pathol 123:25–33
Boncristiani HF et al (2013) In vitro infection of pupae with Israeli acute paralysis virus suggests disturbance of transcriptional homeostasis in honey bees (Apis mellifera). PLoS One 8:e73429
Brooks ME et al (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9:378–400
Calhoun AC et al (2021) Testing the multiple stressor hypothesis: chlorothalonil exposure alters transmission potential of a bumblebee pathogen but not individual host health. Proc R Soc B Biol Sci 288:20202922
Cameron SA, Sadd BM (2020) Global trends in bumble bee health. Annu Rev Entomol 65:209–232
Cameron SA et al (2011) Patterns of widespread decline in North American bumble bees. Proc Natl Acad Sci USA 108:662–667
Cameron SA et al (2016) Test of the invasive pathogen hypothesis of bumble bee decline in North America. Proc Natl Acad Sci USA 113:4386–4391
Cappelle K et al (2016) Israeli Acute Paralysis Virus infection leads to an enhanced RNA interference response and not its suppression in the bumblebee Bombus terrestris. Viruses 8:334
Carrillo-Tripp J et al (2016) In vivo and in vitro infection dynamics of honey bee viruses. Sci Rep 6:22265
Cartar RV (1992) Morphological senescence and longevity: an experiment relating wing wear and life span in foraging wild bumble bees. J Anim Ecol 61:225
Chaimanee V et al (2012) Differential expression of immune genes of adult honey bee (Apis mellifera) after inoculated by Nosema ceranae. J Insect Physiol 58:1090–1095
Chen YP, Pettis JS, Corona M, Chen WP, Li CJ, Spivak M et al (2014) Israeli acute paralysis virus: epidemiology, pathogenesis and implications for honey bee health. PLoS Pathog 10(7):e1004261
Chu CC, Cameron SA (2017) A scientific note on Nosema bombi infection intensity among different castes within a Bombus auricomus nest. Apidologie 48:141–143
Colla SR et al (2006) Plight of the bumble bee: pathogen spillover from commercial to wild populations. Biol Conserv 129:461–467
Core A et al (2012) A new threat to honey bees, the parasitic phorid fly Apocephalus borealis. PLoS One 7:e29639
Dalmon A et al (2021) Possible spillover of pathogens between bee communities foraging on the same floral resource. InSects 12:122
de Miranda JR et al (2021) Protocols for assessing the distribution of pathogens in individual Hymenopteran pollinators. Protoc Exch. https://doi.org/10.21203/rs.3.pex-1453/v1
de Roode JC et al (2008) Virulence-transmission trade-offs and population divergence in virulence in a naturally occurring butterfly parasite. Proc Natl Acad Sci USA 105:7489–7494
Dolezal AG et al (2016) Honey bee viruses in wild bees: Viral prevalence, loads, and experimental inoculation. PLoS One 11:e0166190
Dolgikh VV et al (2002) Peculiarities of metabolism of the microsporidia Nosema grylli during the intracellular development. Parazitologia 36:493–501
Ezenwa VO et al (2010) Hidden consequences of living in a wormy world: nematode-induced immune suppression facilitates tuberculosis invasion in African buffalo. Am Nat 176:613–624
Franzen C (2004) Microsporidia: how can they invade other cells? Trends Parasitol 20:275–279
Franzen C et al (2005) Cell invasion and intracellular fate of Encephalitozoon cuniculi (Microsporidia). Parasitology 130:285–292
Fries I et al (2001) Molecular characterization of Nosema bombi (Microsporidia: Nosematidae) and a note on its sites of infection in Bombus terrestris (Hymenoptera: Apoidea). J Apic Res 40:91–96
Fürst MA et al (2014) Disease associations between honeybees and bumblebees as a threat to wild pollinators. Nature 506:364–366
Galbraith DA et al (2015) Parallel epigenomic and transcriptomic responses to viral infection in honey bees (Apis mellifera). PLoS Pathog 11:1–24
Geffre AC et al (2020) Honey bee virus causes context-dependent changes in host social behavior. Proc Natl Acad Sci USA 117:10406–10413
Goka K et al (2006) Worldwide migration of parasitic mites as a result of bumblebee commercialization. Popul Ecol 48:285–291
Graham AL (2008) Ecological rules governing helminth-microparasite coinfection. Proc Natl Acad Sci USA 105:566–570
Graystock P et al (2015) Parasites in bloom: flowers aid dispersal and transmission of pollinator parasites within and between bee species. Proc R Soc B Biol Sci 282:20151371
Graystock P et al (2016a) The effects of single and mixed infections of Apicystis bombi and Deformed Wing Virus in Bombus terrestris. Parasitology 143:358–365
Graystock P et al (2016b) Do managed bees drive parasite spread and emergence in wild bees? Int J Parasitol Parasites Wildl 5:64–75
Hartgers FC, Yazdanbakhsh M (2006) Co-infection of helminths and malaria: modulation of the immune responses to malaria. Parasite Immunol 28:497–506
Holt RD, Dobson AP (2006) Extending the principles of community ecology to address the epidemiology of host-pathogen systems. Oxford University Press, Oxford
Hoverman JT et al (2013) Does timing matter? How priority effects influence the outcome of parasite interactions within hosts. Oecologia 173:1471–1480
Hsieh EM et al (2020a) Ameliorative effects of phytochemical ingestion on viral infection in honey bees. Insects 11:698
Hsieh EM et al (2020b) Preparation of virus-enriched inoculum for oral infection of honey bees (Apis mellifera). J Vis Exp. https://doi.org/10.3791/61725-v
Hu N et al (2021) Transcriptome analysis reveals changes in silkworm energy metabolism during Nosema bombycis infection. Pestic Biochem Phys 174:104809
Jolles AE et al (2008) Interactions between macroparasites and microparasites drive infection patterns in free-ranging African buffalo. Ecology 89:2239–2250
Karvonen A et al (2019) Importance of sequence and timing in parasite coinfections. Trends Parasitol 35:109–118
Keeling P (2009) Five questions about Microsporidia. PLoS Pathog 5:e1000489
Klinger EG et al (2019) Bombus (Hymenoptera: Apidae) Microcolonies as a tool for biological understanding and pesticide risk assessment. Environ Entomol 48:1249–1259
Lenth R et al (2020) Package ‘emmeans.’ R Packag. version 1.4.734, pp 216–221
Levitt AL et al (2013) Cross-species transmission of honey bee viruses in associated arthropods. Virus Res 176(1–2):232–240
Li W et al (2017) Spore load and immune response of honey bees naturally infected by Nosema ceranae. Parasitol Res 116:3265–3274
Lively CM et al (2014) Interesting open questions in disease ecology and evolution. Am Nat 184(Suppl):S1-8
López-Villavicencio M et al (2007) Multiple infections by the anther smut pathogen are frequent and involve related strains. PLoS Pathog 3:1710–1715
Macías-Macías JO et al (2020) Nosema ceranae causes cellular immunosuppression and interacts with thiamethoxam to increase mortality in the stingless bee Melipona colimana. Sci Rep 10:17021
Manley R et al (2019) Knock-on community impacts of a novel vector: spillover of emerging DWV-B from Varroa-infested honeybees to wild bumblebees. Ecol Lett 22:ele.13323
Martin SJ et al (2012) Global honey bee viral landscape altered by a parasitic mite. Science 336:1304–1306
Masson SWC et al (2017) Mitochondrial glycerol 3-phosphate facilitates bumblebee pre-flight thermogenesis. Sci Rep 7:13107
McIvor CA, Malone LA (1995) Nosema bombi, a microsporidian pathogen of the bumble bee Bombus terrestris (L.). N Z J Zool 22:25–31
McMahon DP et al (2015) A sting in the spit: widespread cross-infection of multiple RNA viruses across wild and managed bees. J Anim Ecol 84:615–624
McNeil DJ et al (2020) Bumble bees in landscapes with abundant floral resources have lower pathogen loads. Sci Rep 10:1–12
Meeus I et al (2011) Effects of invasive parasites on bumble bee declines. Conserv Biol 25:662–671
Menail AH et al (2016) Large pathogen screening reveals first report of Megaselia scalaris (Diptera: Phoridae) parasitizing Apis mellifera intermissa (Hymenoptera: Apidae). J Invertebr Pathol 137:33–37
Moret Y, Schmid-Hempel P (2000) Survival for immunity: the price of immune system activation for bumblebee workers. Science 290:1166–1168
Müller CB, Schmid-Hempel P (1992) Variation in life-history pattern in relation to worker mortality in the bumble-bee, Bombus lucorum. Funct Ecol 6(1):48–56
Müller CB et al (1996) Field evidence that host selection by conopid parasitoids is related to host body size. Insectes Soc 43:227–233
Mwangi TW et al (2006) Malaria and helminth interactions in humans: an epidemiological viewpoint. Ann Trop Med Parasitol 100:551–570
O’Donnell S, Beshers SN (2004) The role of male disease susceptibility in the evolution of haplodiploid insect societies. Proc R Soc B Biol Sci 271:979–983
Otti O, Schmid-Hempel P (2007) Nosema bombi: a pollinator parasite with detrimental fitness effects. J Invertebr Pathol 96:118–124
Otti O, Schmid-Hempel P (2008) A field experiment on the effect of Nosema bombi in colonies of the bumblebee Bombus terrestris. Ecol Entomol 33:577–582
Paris L et al (2018) Effects of the gut parasite Nosema ceranae on honey bee physiology and behavior. Curr Opin Insect Sci 26:149–154
Pedersen AB, Fenton A (2007) Emphasizing the ecology in parasite community ecology. Trends Ecol Evol 22:133–139
Piot N et al (2022) Honey bees and climate explain viral prevalence in wild bee communities on a continental scale. Sci Rep 12:1904
Plischuk S et al (2009) South American native bumblebees (Hymenoptera: Apidae) infected by Nosema ceranae (Microsporidia), an emerging pathogen of honeybees (Apis mellifera). Environ Microbiol Rep 1:131–135
Retschnig G et al (2014) Sex-specific differences in pathogen susceptibility in honey bees (Apis mellifera). PLoS One 9:e85261
Rigaud T et al (2010) Parasite and host assemblages: embracing the reality will improve our knowledge of parasite transmission and virulence. Proc R Soc B Biol Sci 277:3693–3702
Ruiz-González MX, Brown MJF (2006) Males vs workers: testing the assumptions of the haploid susceptibility hypothesis in bumblebees. Behav Ecol Sociobiol 60:501–509
Ryabov EV et al (2014) A virulent strain of deformed wing virus (DWV) of honeybees (Apis mellifera) prevails after Varroa destructor-mediated, or in vitro, transmission. PLOS Pathog 10:e1004230
Sadd BM, Barribeau SM (2013) Heterogeneity in infection outcome: lessons from a bumblebee-trypanosome system. Parasite Immunol 35:339–349
Sadd BM, Schmid-Hempel P (2009a) Perspective: principles of ecological immunology. Evol Appl 2:113–121
Sadd BM, Schmid-Hempel P (2009b) A distinct infection cost associated with trans-generational priming of antibacterial immunity in bumble-bees. Biol Lett 5:798–801
Sandland GJ et al (2007) Interspecific antagonism and virulence in hosts exposed to two parasite species. J Invertebr Pathol 96:43–47
Schneider DS, Ayres JS (2008) Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol 8:889–895
Seppälä O, Jokela J (2016) Do coinfections maintain genetic variation in parasites? Trends Parasitol 32:930–938
Seppälä O et al (2009) Interactions among co-infecting parasite species: a mechanism maintaining genetic variation in parasites? Proc R Soc B Biol Sci 276:691–697
Singh R et al (2010) RNA viruses in Hymenopteran pollinators: Evidence of inter-taxa virus transmission via pollen and potential impact on non-Apis Hymenopteran species. PLoS One 5:e14357
Streicker DG et al (2013) Differential sources of host species heterogeneity influence the transmission and control of multihost parasites. Ecol Lett 16:975–984
Susi H et al (2015) Co-infection alters population dynamics of infectious disease. Nat Commun 6:5975
Telfer S, Bown K (2012) The effects of invasion on parasite dynamics and communities. Funct Ecol 26:1288–1299
Therneau T et al (2015) coxme: mixed effects Cox models. R package version 2.2–5.
Tsaousis AD et al (2008) A novel route for ATP acquisition by the remnant mitochondria of Encephalitozoon cuniculi. Nature 453:553–556
Van Der Steen JJM (2008) Infection and transmission of Nosema bombi in Bombus terrestris colonies and its effect on hibernation, mating and colony founding. Apidologie 39:273–282
Wang H et al (2016) Israeli acute paralysis virus associated paralysis symptoms, viral tissue distribution and dicer-2 induction in bumblebee workers (Bombus terrestris). J Gen Virol 97:1981–1989
Wang H et al (2018) Systemic Israeli acute paralysis virus (IAPV) infection in bumblebees (Bombus terrestris) through feeding and injection. J Invertebr Pathol 151:158–164
Washburn JO et al (2000) Co-infection of Manduca sexta larvae with polydnavirus from Cotesia congregata increases susceptibility to fatal infection by Autographa californica M Nucleopolyhedrovirus. J Insect Physiol 46:179–190
West KL et al (2015) Coinfection and vertical transmission of Brucella and Morbillivirus in a neonatal sperm whale (Physeter macrocephalus) in Hawaii, USA. J Wildl Dis 51:227–232
Wilfert L et al (2016) Honeybee disease: deformed wing virus is a recent global epidemic in honeybees driven by Varroa mites. Science 351:594–597
Yoneda M et al (2008) Commercial colonies of Bombus terrestris (Hymenoptera: Apidae) are reservoirs of the tracheal mite Locustacarus buchneri (Acari: Podapolipidae). Appl Entomol Zool 43:73–76
Acknowledgements
The authors thank Sadd lab members for colony maintenance, especially Toby Bassingthwaite for additional preparation of materials during the coexposure experiment. The authors also thank Dolezal lab members for IAPV inoculum preparation.
Funding
This work was supported by the National Institutes of Health [grant R15 GM129681-01 to B.M.S.], the United States Department of Agriculture [grant 2017-67013-26536 to B.M.S.], two Phi Sigma Biological Society Weigel Grants, one Phi Sigma Mockford-Thompson Fellowship, and one Sigma Xi Grant-in-aid of research to E.C.M. Instrumentation used in this project was funded by a National Science Foundation MRI grant [1725199] with B.M.S. as a co-PI.
Author information
Authors and Affiliations
Contributions
ECM, AGD, and BMS conceived and designed the experiments. ECM performed the experiments. ORC performed microscopy and assisted with data collection. ECM and BMS analyzed the data. ECM and BMS wrote the manuscript; AGD provided editorial advice.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Additional information
Communicated by Andreas Nord.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McCormick, E.C., Cohen, O.R., Dolezal, A.G. et al. Consequences of microsporidian prior exposure for virus infection outcomes and bumble bee host health. Oecologia 202, 325–335 (2023). https://doi.org/10.1007/s00442-023-05394-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-023-05394-x