Abstract
Climate change is increasing water temperature and intensifying the incidence of cyanobacterial blooms worldwide. However, the combined effects of increased temperature and microcystin concentrations as co-stressors on survival and ecological processes in freshwater species are unclear. Here, using purified MC-LR and crude extract of toxigenic Microcystis aeruginosa, we tested the individual and combined effects of three water temperatures (15, 20, 25 °C) and a range of environmentally relevant concentrations of dissolved microcystin and crude extract (0.01–10 µg·L−1) on survival, growth inhibition, grazing and predation rates in three freshwater species: phytoplankton (Scenedesmus quadricauda), zooplankton (Daphnia pulex), and an invertebrate predator (Ischnura elegans). Purified MC-LR exerted a higher growth inhibitory effect on S. quadricauda compared to crude extract with the same concentration of MC-LR, while neither treatment affected its chlorophyll-a content or survival of D. pulex. Crude extract reduced grazing and survival of D. pulex and I. elegans, respectively. The combined effect of higher temperature and crude extract reduced I. elegans survival by 50%. Increased temperature reduced prey handing time in I. elegans by 49%, suggesting a higher predation rate. However, warming together with higher concentrations of crude extract jointly increased zooplankton grazing and reduced damselfly predation. Taken together, these results suggest crude extract, and not necessarily microcystin, can affect survival and productivity in freshwater species, although these effects may vary unevenly across trophic levels. Our findings highlight the importance of complex ecological mechanisms by which warming can exacerbate toxic effects of cyanobacterial bloom extracts on survival and functions among species in eutrophic freshwaters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Harmful cyanobacterial blooms are among the biggest threats to freshwater quality (Brooks et al. 2016), biodiversity and ecosystem functioning globally (Amorim and Moura 2021; Briland et al. 2020; Shahmohamadloo et al. 2020a). Freshwater cyanobacterial blooms have become more frequent and intense, producing microcystins (MCs) and other hazardous cyanotoxins that can cause serious public health, socio-economic and ecological problems in surface waters (Briland et al. 2020; Brooks et al. 2016). This scenario is expected to increase in many lakes due to the complex interactions between multiple stressors associated with anthropogenic climate change, eutrophication, and biological invasions (Schulhof et al. 2019; Sukenik et al. 2015; Urrutia‐Cordero et al. 2020), with UNDESA (2012) proposing an increase by at least 20% in the incidence of harmful algal blooms until 2050. For instance, synergistic interactions between human-induced nutrient enrichment, increased water temperature and thermal stratification have been associated with the intensification of microcystin-producing cyanobacterial blooms in freshwaters (Bui et al. 2018; Harke et al. 2016; Paerl 2008). Although efforts to understand the ecological consequences of microcystin-producing blooms on freshwater ecosystems have been considerable (Ibelings et al. 2008; Wang et al. 2017), one major unanswered question remains the ecological importance of cyanotoxins relative to other stressors in the effects observed during freshwater harmful blooms (Briland et al. 2020; Ibelings et al. 2008). Therefore, it is unclear how exposure to increased cyanotoxin concentrations may affect survival of freshwater species and the ecosystem processes in which they are involved.
MCs are the most prominent group of cyanotoxins produced by several bloom-forming cyanobacteria in freshwaters (Chorus and Welker 2021; de Figueiredo et al. 2004; Janssen 2019). They occur naturally either as cell-bound, intracellular toxins or as dissolved extracellular toxins in freshwater bodies (Pham and Utsumi 2018). The intracellular MC content in intact cyanobacterial cells is usually several orders of magnitude higher than dissolved levels in water bodies, where concentrations rarely exceed 10 µg/L (Chen et al. 2017; Zhu et al. 2021). MC occurrence in surface waters has been linked to severe adverse effects in humans and a wide range of animals, such as birds, amphibians, fish, and zooplankton (Chen et al. 2009; Jos et al. 2005). Therefore, the World Health Organisation (WHO) recently recommended provisional guideline values of 1 µg/L and 12 µg/L as lifetime and short-term permissible MC-LR concentrations in drinking, and 24 µg/L for recreational waters (Chorus and Welker 2021). A few reports suggest that occasional occurrence of dissolved MC concentrations in surface waters can reach > 1 mg/L in specific situations, such as during cell lysis of senescent blooms, or immediately after algicide treatment (Babica et al. 2007; Kotak and Zurawell 2007; Sivonen and Jones 1999). However, other studies have shown rapid degradation of dissolved MC concentrations, such as > 50% within 5 days (Zheng et al. 2004). This rapid degradation suggests that high concentrations of dissolved MCs are unlikely to persist for longer periods in freshwaters and so typical environmental concentrations of dissolved MCs rarely exceed the above-mentioned guideline values recommended for human exposure (Chen et al. 2017; Chorus and Welker 2021). However, it is unclear whether the guideline values for human exposure can serve to estimate risks to populations of aquatic organisms. Hence, it is important to understand the effects of low concentrations of dissolved microcystins on survival and functions among freshwater species.
Freshwater species mediate important ecological processes, such as primary production, herbivory, and predation in aquatic ecosystems (Galic et al. 2017). The dynamics of these processes have been associated with the overall productivity and functioning in aquatic systems (McKie et al. 2009; Oliver et al. 2015). However, evidence of the impact of dissolved MCs at sublethal concentrations on freshwater species is yet controversial and may potentially influence their survival and function (Burkholder et al. 2018; Chen et al. 2005; Shahmohamadloo et al. 2020b). Although several explanations have been proposed regarding the ecological role of MC production in competition (Babica and Maršalek 2006) and cyanobacteria–zooplankton interactions (Ger et al. 2016; Omidi et al. 2018), existing empirical evidence on these propositions is still currently limited and contradictory (Moustaka-Gouni and Sommer 2020). Previous studies have linked MC exposure with a variety of adverse effects in different freshwater taxa, including allelopathic inhibition of growth and photosynthetic pigments in algae (El-Sheekh et al. 2010), altered feeding and life history in zooplankton (Ghadouani et al. 2004; Smutná et al. 2014), and bioaccumulation and oxidative stress in molluscs (Zhang et al. 2016). These studies demonstrated the effects of either intact cells, crude extracts, or purified toxin on single species at concentrations well above those typically found in freshwaters (Burkholder et al. 2018). Therefore, findings from such studies may be difficult to extrapolate to the field as inferences drawn from single-species or single-stressor studies may be inadequate for generalising to more complex situations (Edwards and Pascoe 2018).
Climate change has been predicted to increase water temperature and thermal stratification for many lakes (Urrutia‐Cordero et al. 2020). Consequently, increased water temperature may act independently (Woodward et al. 2010) or in conjunction with other stressors, such as dissolved MC concentrations, to affect survival and key functions among aquatic species (Burkholder et al. 2018). Increased water temperature will accelerate rates of metabolic and cellular activities in freshwater organisms and such effects may vary across species depending on species-specific thermal thresholds (Brown et al. 2004). In combination with a second stressor, this change in metabolism may result in non-linear synergistic or antagonistic effects which may lead to unexpected ecological outcomes at the community level (Vinebrooke et al. 2004), potentially influencing trophic interactions (Woodward et al. 2010).
To investigate the potential impacts of warming and cyanotoxins on species survival and trophic interactions, we tested the combined effects of temperature and dissolved MC concentrations (both using purified MC-LR and crude extract of Microcystis aeruginosa) on survival and processes relating to resource–consumer interactions in three freshwater taxa: Scenedesmus quadricauda (green alga), Daphnia pulex (zooplankton grazer) and Ischnura elegans (predatory damselfly larva). These species occupy significant positions in the freshwater food webs, and have been widely used as sentinel experimental models in many ecotoxicological studies (Šulčius et al. 2017). We conducted a suite of factorial laboratory experiments, consisting of three temperatures (15 °C, 20 °C, 25 °C) and two MC treatments (purified MC-LR and crude extract) at environmentally realistic concentrations (0.01–10 µg/L). The temperature range tested is in accordance with those reported in previous studies, corresponding to characteristic average (15 °C), slightly elevated (20 °C), and elevated (25 °C) thermal conditions in European freshwaters (Henry et al. 2017). MC concentrations tested were in accordance with existing evidence showing that dissolved MCs typically occur at relatively low concentrations ranging from 10 s of ng/L to a few µg/L in water bodies (Chen et al. 2013; Wei et al. 2020). We monitored effects of both stressors on (i) primary productivity in the algal species by measuring algal growth inhibition and photosynthetic chlorophyll-a; (ii) survival and grazing rate in a daphnid species; and (iii) survival and predation rates in damselfly larva.
First, based on previous studies (Burkholder et al. 2018), we expected negative effects of sublethal MC concentrations on survival, primary production, grazing and predation in our study species. This is because existing studies have associated exposure to low concentrations of chemical stressors below recommended safe thresholds with non-linear patterns of negative effects on aquatic species (de Souza Machado et al. 2017; Rivetti et al. 2016). Therefore, we refer to our chosen concentrations as “low” relative to the experimental concentrations used in previous studies and relative to short-term environmental concentrations sometimes observed at the peak of bloom senescence (Chen et al. 2005; DeMott et al. 1991; Shahmohamadloo et al. 2020a). Second, we hypothesised that survival, grazing and predation would be reduced for the zooplankton and predator species at higher temperatures when thermal thresholds are exceeded (Heugens et al. 2003; Kenna et al. 2017; Verheyen et al. 2019). Finally, we predicted interactive effects of temperature and dissolved MCs on survival and feeding-related processes in our study species, such that negative effects of toxins are exacerbated at higher temperatures. This third hypothesis is based on increased metabolic rates at higher temperatures, leading to greater active uptake and metabolism of the toxin (Zurawell et al. 2005).
Materials and methods
Cyanobacterial culture
A toxigenic cyanobacterial strain, Microcystis aeruginosa, (CCAP/14/50/16) was obtained from the Culture Collection for Algae and Protozoa (CCAP), Scottish Marine Institute, Scotland. M. aeruginosa was cultivated in the laboratory as batch cultures under axenic conditions in 250 mL Erlenmeyer flasks containing 150 mL autoclaved BG-11 culture media prepared in line with the CCAP’s recipe. These cultures were maintained under constant temperature (25 ± 1 ºC), light intensity (25 µmol quanta m−2 s−1) and photoperiod cycle (12 h: 12 h light/dark) in a shaking incubator until cells were harvested. Before harvest, daily cell density was monitored by spectrophotometric measurement of the optical density (OD) at 680 nm as well as by light microscopy using a compound microscope and Sedgewick-Rafter Counting Chamber. The relationship between the daily spectrophotometric OD measurement and the cell density was established by fitted linear regression (R2 = 0.97). Cells were harvested at the stationary phase and cell density was estimated by spectrophotometry before the cells were lysed through a freeze–thaw cycle to release the intracellularly bound MC content (note that this may potentially release other unidentified water-soluble cellular components). The total MC content in the crude Microcystis extract was quantified and expressed as MC-LR using a semi-quantitative Adda-specific MC ELISA kit (CAT No. ALX-850–391-KI01, Enzo Life Sciences) according to Sarnelle et al. (2010). The purified MC-LR (CAS No. 101043–37-2, purity ≥ 95%) was supplied by Cayman Chemical Company, UK.
Algal primary production and photosynthetic chlorophyll-a
Scenedesmus quadricauda (A950) was purchased from Sciento Scientific Ltd., Manchester, UK and maintained in BG-11 media on a cool white fluorescent lamp-illuminated shelf in a controlled temperature room kept at 21 ± 1 °C; 54 µmol quanta m−2 s−1 and 14 h:8 h light/dark photoperiod. Daily cell density increases were monitored by spectrophotometric measurement of the optical density (OD) at 680 nm and using a compound microscope and Sedgewick-Rafter Counting Chamber.
We set up 40 experimental replicates consisting of 100 mL cultures of S. quadricauda in 150 mL Erlenmeyer flasks for each temperature, in accordance with the OECD guidelines (OECD 2011). In all, a total of 120 replicates across three temperatures (15, 20 and 25 °C) was established. Each replicate culture was made up of sterile BG-11 medium, a known concentration of MC-LR (either in the crude Microcystis extract or the purified toxin) and 10 mL of the S. quadricauda inoculum (2.64 × 104 cells/mL) which had been incubated and acclimated as an exponentially growing culture for 4 days before the experiment. We tested the effects of five concentrations of the purified MC-LR treatment (0.01, 0.1, 0.5, 1.0 and 10.0 µg/L) and the equivalent concentrations in crude Microcystis extracts on the growth inhibition and photosynthetic pigments content of S. quadricauda at three different temperatures: 15, 20 and 25 °C. Note that the crude Microcystis extract represents a more ecologically relevant mixture of secondary metabolites released during cell lysis including MC. Throughout the study, we express the crude extract concentration in terms of the concentration of MC that it contains. Each treatment concentration and control had four replicates. The control group consisted of a blank control and a solvent control. The blank control consisted of all the experimental conditions without MC treatment, while the solvent control also contained 0.1 mL of methanol (0.1%, v/v) to distinguish the effects of the organic solvent used as diluent from the effects of the purified toxin. The experiment was incubated at three temperatures (15, 20 and 25 °C) and constant light intensity (54 µmol quanta m−2 s−1) over a fixed photoperiod (14 h:8 h hour light/dark cycle) for 72 h. On each day of the experiment, 5 mL of the samples was taken and replaced with equal volume of the growth medium. The daily biomass increase for each replicate was estimated from the linear regression between the spectrophotometric OD680 data and the microscopic cell counts (Ma et al. 2015). The percentage growth inhibition of S. quadricauda was calculated from the specific growth rate of the treatment relative to the solvent control (Wang et al. 2017). The effects of temperature and MC treatments on chlorophyll-a content as a surrogate measure of photosynthetic processes were determined by taking the spectrophotometric optical density reading of the methanol extracted samples at OD440, OD645, OD652 and OD663. The pigment content was calculated as described by Fang et al. (2018).
Zooplankton survival and grazing
Adults of the cladoceran zooplankton, Daphnia pulex, were obtained from Blades Biological Ltd., Kent, UK. Daphnids were cultured in a controlled temperature (CT) room at 21 ± 1 °C and 16 h:8 h light:dark photoperiod for 3 weeks, until third broods of neonates were produced by each adult female. Fifteen adult animals were maintained in 500 mL glass beakers filled with 300 mL of “Aachener Daphnien Medium” (ADaM), a modified artificial media for zooplankton (Klüttgen et al. 1994). Daphnids were fed with 10 mL of S. quadricauda (5.0 × 106 cells per mL) three times weekly. The culture medium and algal food material were renewed every 3 days and the neonates produced were counted and removed daily (Rohrlack et al. 2001). To ensure standardised maternal conditions, only neonates produced at the third brood and after were used for experiments.
A 48-h static acute toxicity test was conducted to test the combined effects of temperature and MC treatments on the survival of D. pulex following the OECD guidelines (OECD 2004). Daphnid neonates less than 24 h old and taken from the female’s third brood were exposed to five treatment concentrations (0.01, 0.1, 0.5, 1.0, and 10.0 µg/L) of purified MC-LR and three concentrations of crude Microcystis extract (0.01, 0.1, and 0.5 µg/L). Tests were conducted in 100 mL plastic beakers, containing 50 mL of the experimental medium (ADaM), MC treatments and 10 daphnid neonates per cup. Daphnids were incubated under controlled conditions at three different temperatures (15, 20, and 25 °C), 54 µmol quanta m−2 s−1 and 14 h:10 h hour light/dark cycle. Six replicates of a control, consisting of the same experimental conditions without MC treatments, were kept at each temperature, while three replicates per MC concentration were tested at each temperature (42 replicates in total, see Table S1 for a schematic of the experimental designs). The proportion of immobilised neonates per replicate was counted as a surrogate measure of mortality (endpoint) at 24 and 48 h (Luo et al. 2018).
The grazing inhibition tests in D. pulex were conducted in accordance with the method described by Jesus et al. (2014). Five 4-day-old daphnids were exposed to the same concentrations of either MC-LR or crude Microcystis extract as those used for the survival experiments at the same three temperatures (15, 20, and 25 °C) in 50 mL plastic beakers. Each beaker contained a 20 mL solution made up of a combination of the test medium (ADaM), MC treatments, and 5 mL of Scenedesmus culture (1.45 × 106 cells/mL) as a food source for daphnids. We used two experimental treatments without MC: the blank treatment experienced the same experimental conditions as the MC replicates but without daphnids or MC to measure algal growth in the absence of grazing and MC. The control treatment experienced the same experimental conditions as the MC replicates and included five daphnids but no MC treatments were added to measure grazing in the absence of MC. These experimental conditions were all made in four replicates across the control, the blank and each combination of MC concentration and temperature. To reduce the influence of light on algal growth during the experiment, the tests were incubated in the dark while animals were only allowed to graze for 24 h. At the end of the test, daphnids were gently removed with the aid of a plastic pipette, while individual beakers were shaken vigorously to ensure uniform concentration of algae before determining the cell density by taking the spectrophotometric optical density (OD680) at 680 nm. The individual grazing rate for each animal was estimated relative to the blank control and expressed as the change in the cell density during the 24 h grazing test (Allen et al. 1995).
Damselfly survival and predation
Blue-tailed damselfly larvae, Ischnura elegans, were collected between June and July 2019 from ponds located at None-Go-By Farm (53° 52′ 33.75'' N, 1° 38′ 37.57'' W) in Horsforth, West Yorkshire, UK. Larvae were sampled using a pond net, sorted in a plastic tray, and transported to the laboratory in a small cool box containing pond water for further identification using taxonomic keys (Cham 2012). Ischnura elegans were acclimated to laboratory conditions in plastic tanks containing 100 mL of aged, dechlorinated tap water, maintained at constant temperature of 20 °C and 14 h:10 h light:dark photoperiod cycle. Larvae were fed ad libitum with Daphnia magna, while a wooden dowel rod was used to provide a perch for the larvae in each tank (Villalobos-Jiménez et al. 2017).
A 24 h functional response experiment was conducted to test the effects of MC and temperature on survival and prey consumption using methods in Villalobos-Jiménez et al. (2017). Larvae with a size range of 1.5–3.1 mm at the 9th–11th instars were acclimated to the experimental temperature and starved for 24 h before the start of the experiment. Animals were housed individually in 200 ml plastic containers in aged, dechlorinated tap water, incubated at 14 h:10 h light:dark photoperiod cycle, and exposed to two concentrations of crude extract treatments (0.05 and 0.2 µg/L) or a control containing no extract at two different temperatures (15 and 25 °C). Data were also collected at 20 °C, but due to a lack of size-matched individuals, those data were not analysed. Purified toxin was not available for this experiment. Individual larvae were fed with one of five different prey densities, consisting of 5, 10, 15, 30, and 50 D. magna individuals (body size 1.0–1.4 mm) as prey. Seven replicates of each prey density were used for the experiment at 15 °C and 25 °C. Each damselfly was only used in one replicate. At the end of the 24 h exposure, the numbers of Daphnia consumed were counted.
Data analysis
Algal response data (growth inhibition and chlorophyll-a pigment) were checked for normality assumptions using the Shapiro–Wilk test. The 72 h percentage algal growth inhibition data were log-transformed to avoid non-normal distribution of the residuals and general linear models were fitted to test the effects of toxin and temperature on both response variables.
Survival of D. pulex individuals exposed to MC treatments were found to be greater than 50%, therefore survival of daphnids could not be estimated as the median lethal concentrations (LC50); a concentration that would kill 50% of the exposed population. Instead, Cox proportional hazard (CPH) models in the survival (Therneau 2015; Therneau and Grambsch 2000) and survminer (Alboukadel et al. 2019) packages in R were fitted to test for variation in time to death across toxin and temperature treatments. The 24 h grazing in zooplankton was checked for normality assumptions and analysed by fitting general linear models. Significant differences among treatment and temperature levels were determined using Tukey’s multiple comparison test in the multcomp package in R (Hothorn et al. 2008).
The individual and combined effects of temperature and crude Microcystis extract treatments on the survival of I. elegans during the 24 h experiment were tested by fitting a binary logistic regression. We explored the differences in the effects of temperature and crude extract treatments on the attack rate and handling time of I. elegans using the frair package (Pritchard 2017; Pritchard et al. 2017) in R with a Benjamini–Hochberg correction to the resulting p values. Prey consumption rates followed a type II functional response. Since there was no prey replacement during the experiment, we estimated handling time (h) and attack rate (a) following Rogers’ type II formula (Rogers 1972) with Lambert’s W function (Bolker 2008). We tested the difference in the handling times and attack rates between treatments using bootstrapping.
Results
Algal growth inhibition and chlorophyll-a
Both purified MC-LR and crude extract containing a corresponding amount of MC-LR inhibited the growth of S. quadricauda by 20–30% to a similar extent at all concentrations tested (Fig. 1A; Table 1). The effect of pure MC-LR was slightly but significantly more pronounced than that of crude extract containing the same concentration of MC-LR (p = 0.001; Fig. 1A; Table 1). Inhibition was significantly greater at 25 °C than at the two lower temperatures tested (p < 0.001; Fig. 1A).
Effect of temperature and treatment concentration on A growth inhibition and B chlorophyll-a in the freshwater alga Scenedesmus quadricauda and survival (C) and grazing rate (D) in the zooplankton Daphnia pulex. Note that crude extracts contained the same concentration of MC-LR as the treatment with purified MC-LR and that each point represents mean ± SEM error bars in A, B and D but mean ± 95% binomial confidence levels error bars in C. For sample sizes in each experiment, please see Table S1
Our results showed no evidence of statistically significant effects of purified MC-LR (p = 0.180), crude Microcystis extract (p = 0.109) or toxin concentration (p = 0.131) on the chlorophyll-a pigment content of S. quadricauda (Fig. 1B; Table 1). However, increased water temperature from 15 °C to 25 °C significantly reduced chlorophyll-a pigment content of S. quadricauda (p < 0.001; Fig. 1B). The highest chlorophyll-a content in this study was observed at 15 °C (p < 0.001; Fig. 1B).
Zooplankton survival and grazing
Neither purified MC-LR nor crude Microcystis extract treatments had significant effects on the survival of D. pulex at the range of concentrations tested in this study when compared with the control (Table 2, Fig. 1C). However, increased water temperature from 15 °C to 25 °C significantly reduced survival of D. pulex individuals (p < 0.001; Table 2, Fig. 1C). Crude Microcystis extract only (p = 0.045), but not the purified MC-LR (p = 0.280), significantly reduced the grazing rate of D. pulex relative to the control (Table 1, Fig. 1D). However, these effects did not differ significantly across the range of toxin concentrations tested in this study (p = 0.562; Table 1, Fig. 1D). Interestingly, the crude extract increased the grazing rate of D. pulex relative to the control (p = 0.025, Fig. 1D), albeit without a trend across treatment concentrations and temperature.
Damselfly survival and predation
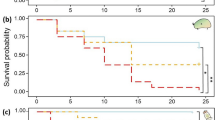
The crude Microcystis extract treatment (from 0.05 µg/L to 0.2 µg/L) significantly reduced survival of I. elegans relative to the control, albeit only at 25 °C and not at 15 °C (p = 0.036; Fig. 2A, Table 2). The observed effect was far more pronounced in the crude extract treatment containing the higher concentration of MC-LR. Overall, the survival odds ratio of I. elegans in the control was approximately 6 times higher than in presence of the extract (Table 2). Although increased temperature alone had no significant overall effect on the survival of I. elegans (p = 0.512; Fig. 2A, Table 2), there was a significant interaction between increased temperature and crude extract, such that the highest extract concentration containing 0.2 µg/L MC-LR and increased temperature at 25 °C jointly reduced survival of I. elegans by almost 50% compared to the control (p = 0.007; Fig. 2A, Table 2).
Combined effects of temperature and crude extract on survival and predatory functional response of damselfly larvae, I. elegans. A Survival (circles = 15 °C and triangles = 25 °C) and error bars represent 95% confidence levels. B Predation at 15 °C (grey = 0 µg/L, blue = 0.05, red = 0.2 µg/L), with considerable overlap. C Predation at 25 °C (grey = 0 µg/L, blue = 0.05, red = 0.2 µg/L), where the grey control has a higher predation rate than the 0.05 and 0.2 µg/L treatments. In B and C, the shaded areas represent 95% confidence levels. B and C are functional response plots, showing the number of prey consumed when those prey are presented to a predator at a range of densities. If a predator was able to consume all prey regardless of density, there would be a 1:1 relationship while deviation from the 1:1 relationship indicates a limiting effect of attack rate or handling time. For sample sizes in each experiment, please see Table S1
Figure 2B, C shows the functional response curves resulting from experiments at 15 °C and 25 °C, respectively, under control (0 µg/L), 0.05 µg/L and 0.2 µg/L of crude extract. In each plot, a curve closer to the 1:1 line indicates higher rates of predation (predator consumes a greater proportion of available prey in the time period) that may correspond to higher attack rates, lower handling times, or both. In the absence of crude extract, there was no difference in the attack rate of I. elegans between the two temperatures (p = 0.92; Table 3). However, the prey handling time of I. elegans was significantly reduced by 49% when temperature increased to 25 °C, indicating a higher predation rate (p < 0.001; Table 3; note the higher asymptote on the grey control line in Fig. 2C compared to Fig. 2B). When looking only at the 15 °C experimental treatments, functional response curves showed no significant effects of increased crude extract concentrations compared to the control on the attack rate (control vs 0.05 µg/L: p = 0.67; control vs 0.2 µg/L: p = 0.45) or prey handling time of I. elegans (control vs 0.05 µg/L: p = 0.64; control vs 0.2 µg/L: p = 0.92; Table 3; note the overlapping confidence intervals in Fig. 2B). At 25 °C, a crude extract concentration corresponding to 0.2 µg/L MC-LR significantly increased the prey handling time compared to the control at 25 °C (p < 0.001; Table 3; note the higher predation rate indicated by the grey control curve compared to the red 0.2 µg/L curve in Fig. 2C). No significant effect was observed on the attack rate of I. elegans under increased crude extract concentration at 25 °C (p = 0.38; Table 3).
Discussion
This study tested the combined effects of increased environmental temperature and dissolved MC concentrations in purified MC-LR and crude extract treatments on survival, productivity, and resource–consumer interactions in three important freshwater species covering three trophic levels. Our results showed increased crude extract concentrations and elevated temperature jointly reduced the survival of I. elegans, while the predation rate of I. elegans increased only at higher temperatures. Increased temperature in combination with increased crude extract concentrations reduced predation rate in I. elegans, but increased grazing rate in D. pulex. Independently, increased temperature inhibited algal growth and photosynthetic chlorophyll-a pigment in S. quadricauda and reduced survival of D. pulex. However, purified MC-LR demonstrated a slight, but statistically significant higher inhibitory effect on the growth of S. quadricauda compared to crude extract with corresponding MC-LR concentrations. Taken together, our results suggest that the observed negative effects of our crude extract treatment in this study may not be entirely due to the dissolved MC concentrations alone. The potential effects of other unidentified secondary metabolites and their combined effects with one another and temperature cannot be ruled out.
Effect of low environmental MC-LR concentrations
We observed a significant inhibitory effect of purified MC-LR on the growth of S. quadricauda while the photosynthetic chlorophyll-a content was unaffected by either crude extract or purified MC-LR treatments. This high inhibitory effect of the purified MC-LR on the growth of S. quadricauda is accordance with previous findings in the literature (Babica et al. 2007; El-Sheekh et al. 2010; Pflugmacher 2002) and supports the argument that MC may potentially be involved in the allelopathic interactions between cyanobacteria and other phytoplankton during interspecific competition (Omidi et al. 2018). Several studies have proposed that MC may act as a potential allelochemical (Babica and Maršalek 2006; Pflugmacher 2002), and Figueredo et al. (2007) have suggested that the toxin may be capable of shaping phytoplankton succession and community structure during freshwater blooms. The putative inhibitory role of MCs in allelopathic interactions has been attributed to its potential to inhibit growth, photosynthesis, and induce cellular oxidative stress during interspecific competition (Bittencourt-Oliveira et al. 2015; Omidi et al. 2018). However, the complex mechanisms behind these adverse effects are yet to be fully understood as evidence from previous laboratory studies indicate contrasting effects across MC treatments (Babica and Maršalek 2006; Ma et al. 2015).
Dissolved MC concentrations in purified toxins or crude extracts have been associated with a variety of adverse effects in zooplankton and aquatic invertebrates (Bownik 2016; Burkholder et al. 2018; Ger et al. 2016). However, in this study, low concentrations of the purified MC-LR had no effect on survival and grazing rate of D. pulex, but crude extract containing corresponding MC-LR concentrations exerted significant negative effects. This finding is in accordance with the observations of Jungmann (1994), suggesting that the negative effects observed in the crude extract were possibly not due to dissolved MC-LR concentrations but potentially other unidentified metabolites. The observed tolerance of D. pulex to purified MC-LR concentrations is in agreement with previous studies (Dao et al. 2018, 2010; Lürling and van der Grinten 2003), that reported little or no adverse effects of the purified toxin on survival of Daphnia at ecologically relevant concentrations. Lürling and van der Grinten (2003) and Chen et al. (2005) found no significant effects on survival of D. magna after a 21-day exposure to dissolved concentrations as low as 3.5 µg/L and 10 µg/L of the purified MC-LR, respectively. However, a longer term exposure to 5 µg/L and 50 µg/L of the purified MC-LR in the laboratory resulted in 10 and 60% reductions in survival of Daphnia, respectively, after 60 days (Dao et al. 2010). The range of the purified MC-LR concentrations (1–10 µg/L) tested in this study is several orders of magnitude lower than the lethal concentrations (LC50) reported for the purified toxin in Daphnia (Bui et al. 2020; DeMott et al. 1991). Moreover, there is increasing evidence that dissolved MC concentrations that persist in surface waters are usually < 10 µg/L (Graham et al. 2020; Loftin et al. 2016; Lürling and van der Grinten 2003; Skafi et al. 2021). Lürling and van der Grinten (2003) reported a range of 0.2 µg/L to 4.7 µg/L, which may persist for ~ 10 weeks depending on the predominant variant (Lahti et al. 1997). Although dissolved MC concentrations may be higher and tend to persist longer in tropical waters, where harmful cyanobacterial blooms dominate for longer period due to warmer temperature and eutrophication (Mowe et al. 2015). Hence, our results suggest low concentrations of the dissolved MCs are unlikely to cause acute toxicity in aquatic organisms, with a potentially greater role for other secondary metabolites released during cell lysis. Nevertheless, prolonged exposure in the environment may exert subtle chronic effects on individual survival, physiological fitness and key processes (Dao et al. 2018, 2010), which may propagate to higher levels of biological organisation (Nilsen et al. 2019).
Effect of crude extract containing low concentrations of MC-LR
Growth inhibitory effect of crude Microcystis extract on S. quadricauda in this study was relatively low compared to the effect of purified MC-LR treatments, even though the concentration of MC-LR in the crude extract was the same as in the purified MC-LR treatments. This may be due to the nature of the crude extract and possibly attributed to the fact that the effects of MC-LR might have been masked by other unidentified co-occurring metabolites present in the crude extract (Janssen 2019). Crude cyanobacterial extracts have been shown to consist of a variety of heterogeneous metabolites produced by cyanobacteria other than MCs (Janssen 2019). The combined effects (be they additive, synergistic, or antagonistic) of these heterogeneous metabolites can mask or modulate potential adverse effects on aquatic organisms (Ibelings et al. 2008; Janssen 2019). However, contrary to our expectation, neither treatment affected chlorophyll-a content in our study. The quantification of chlorophyll-a and other photosynthetic pigments represents an important biomarker of photosynthetic and respiratory rates in algae and have been widely used to evaluate sensitivity of algae to xenobiotic exposures (Fang et al. 2018; Li et al. 2005). As reported in previous studies (Cheng et al. 2015; Wei et al. 2010), photosynthetic pigmentation is expected to serve as an early warning and a more sensitive signal to MC stress compared to growth inhibition. This is because changes in photosynthetic pigments are believed to occur earlier at the molecular level of the cells than growth inhibition during exposure to chemical stressors (Fang et al. 2018). However, in contrast to this expectation, our results indicate that low environmental MC concentrations in the range of those tested in this study are unlikely to affect the ability of algae to synthesise chlorophyll-a pigments during blooms. This observation could be due to the short duration of exposure in the present study, and we cannot rule out the fact that adverse effects on photosynthetic pigments might yet be observed as a consequence of a longer period of exposure.
In addition, the crude Microcystis extract treatments used in this study showed varied effects on survival and feeding in D. pulex and I. elegans. The observed variations in species’ sensitivity to concentrations of crude extract among these experimental models may be explained by differences in assimilation, metabolism and detoxification mechanisms in these organisms (Kozlowsky-Suzuki et al. 2012).
Effects of increased environmental temperatures
Increased environmental temperature was associated with reduced survival and productivity in our model organisms. In support of the second hypothesis in this study, increased temperature reduced growth and photosynthetic chlorophyll-a content of S. quadricauda. The effect of increasing temperature was non-linear and followed a U-shaped dose–response pattern in the purified MC-LR treatments, possibly suggesting evidence of temperature-mediated hormesis. Hormesis has become an increasingly reported biphasic evolutionary phenomenon in many organisms (Erofeeva 2022), where low-dose exposure to environmental stress induces stimulation and high-dose inhibition, due to increased resilience or overcompensation (Agathokleous et al. 2019; Calabrese 2008; Forbes 2000). Increasing evidence has shown hormesis as an important adaptive biological response to a wide range of abiotic (including, temperature and light) and biotic natural stressors (cyanotoxins and allelochemicals) as well as anthropogenic pollutants in green plants and algae (Agathokleous 2021; Agathokleous et al. 2019; Erofeeva 2022). Here, our data consistently showed a U-shaped, non-linear effect across the range of low purified MC-LR concentrations tested, suggesting that increased temperature may have brought about a hormetic response of chlorophyll-a pigment to low concentrations of the purified MC-LR. These results are consistent with previous findings on temperature-mediated hormesis in plants (Agathokleous 2021; Agathokleous et al. 2019; Erofeeva 2022) and the effects of environmental warming on algae (Chalifour et al. 2014; Chalifour and Juneau 2011; Gomes and Juneau 2017; Larras et al. 2013). While warming beyond optimum thermal thresholds can induce adverse physiological changes at molecular (pigment alterations) and cellular levels (growth inhibition) in algae (Gomes and Juneau 2017), such effects are unlikely to be present in our study as our highest temperature (25 °C) is a standard culturing temperature and reductions in population growth rate only occur > 32 °C (Zargar et al. 2006). As a result, apparent negative effects of temperature are more likely due to interactions with cyanobacterial products.
Increased temperature had no effect on survival but was associated with reduced prey handling time of I. elegans, thereby increasing its predation rate. The observed thermal tolerance at 25 °C among I. elegans larvae could be due to the short exposure duration (24 h) in our experiment. Arguably, the exposure conditions and duration used in this study were possibly within the thermal preference for this freshwater predator. This finding is consistent with previous studies showing increased survival rate (Carbonell and Stoks 2020; Dinh et al., 2013) and thermal tolerance, CTmax (de Beeck et al. 2017), in I. elegans larvae exposed to a range of thermal conditions (20–24 °C) for more than 6 days in the laboratory. However, while 25 °C is considered an elevated thermal condition in the present study, our data suggests that this temperature is unlikely to be stressful for I. elegans. Carbonell and Stoks (2020) found a significant reduction in the survival of I. elegans exposed to 28 °C and above for 10 days, suggesting a longer term exposure to higher temperatures may be associated with significant adverse effects on survival of I. elegans. Moreover, our findings on increased predation rate in I. elegans at high temperature in this study are consistent with earlier studies (Thompson 1978; Wang et al. 2020), and may presumably be due to increased metabolic rate at higher temperature (Brown et al. 2004). Elevated temperature has been shown to accelerate the rate of metabolic processes, energy requirement and food intake in ectotherms (Brown et al. 2004; Galic et al. 2017; Stoks et al. 2014). Hence, higher predation rates observed in I. elegans at higher temperatures in this study may be associated with potential beneficial effects, including increased growth rate and immune response of the damselfly predator (Van Dievel et al. 2017; Wang et al. 2020).
We also observed reduced survival and grazing rates in D. pulex at higher temperatures. Our data in this study corroborate earlier findings by Müller et al. (2018), who demonstrated reduced survival among D. magna populations at temperatures above 29 °C, and reduced filtration rate in Daphnia when the optimal temperature (20 °C) was exceeded. Hence, these results suggest that the abundance of these keystone species may potentially be at risk as the climate becomes warmer. More importantly, vital processes that underpin ecosystem functions among key freshwater species may become impaired unless other more thermotolerant filter-feeders fill the gap as an expected ecosystem response (Ger et al. 2016).
Combined effects of environmental temperature and crude extract concentrations
More ecologically significant and environmentally realistic findings than individual effects of temperature and toxins are those relating to their combined effects on freshwater species. Here, the interaction between the crude extract and increased temperature was associated with reduced survival of I. elegans. Higher temperatures lead to increases in metabolic activity and accelerate the toxicokinetics of chemical stressors in ectotherms (Brown et al. 2004; Noyes et al. 2009). Increased metabolic activity at higher temperatures presumably elevated the rate of uptake, assimilation, and toxicity of the bioactive compounds in the crude extract (Buchwalter et al. 2003; Kim et al. 2014; Kozlowsky-Suzuki et al. 2012). Such temperature-mediated toxicity could be responsible for the 50% decline observed in the survival of I. elegans exposed to high concentration of the crude extract at 25 °C in our study. Here, our finding supports the hypothesis on the combined effects of temperature and crude extract and corroborates earlier studies that demonstrated their combined effects on freshwater species (Kim et al. 2014; Lamb et al. 2019; Xiang et al. 2017). Hence, the present study suggests that warming may co-occur with a much broad range of cyanobacterial metabolites following bloom senescence leading to impaired survival and feeding among important species (Paerl and Huisman 2008; Walls et al. 2018).
We find evidence for antagonistic interactions between the effects of crude extract concentrations and increased temperature on the grazing rate of D. pulex and the predation rate of I. elegans. While increased temperature alone reduced prey handling time in I. elegans, the two stressors in combination jointly increased the prey handling time to a greater extent. Similarly, the combined effects of crude extract and elevated temperature further increased the grazing rates of D. pulex. Earlier studies have shown synergistic interactions between MCs and other environmental stressors may occur at lower dissolved MC concentrations, while antagonistic interactions were mostly observed at higher dissolved MC concentrations in other freshwater species (Liang et al. 2017, 2018; Wei et al. 2020). However, as dissolved MCs rarely occur in high concentrations under natural conditions (see above), a more realistic scenario for toxic blooms in freshwater bodies will be a combination of low concentrations of cyanobacterial metabolites with increased environmental warming.
Conclusion
Here, we demonstrate using a suite of laboratory microcosms that increased temperature and environmentally relevant exposure to purified MC-LR, but more so to crude cyanobacterial extract containing MCs as well as other unidentified compounds, can affect survival and feeding in key freshwater species. We showed that low environmental MC concentrations adjudged to be safe for human health by the WHO can have species-specific effects for survival and ecosystem functions in S. quadricauda and possibly, as component of crude extract, in further freshwater taxa. Importantly, a more ecologically relevant effect on freshwater communities may become apparent when exposure to low concentrations of complex mixtures of cyanobacterial metabolites in the environment coincides with increased water temperature in eutrophic waters. Our results build on existing knowledge that suggests that other yet unidentified bioactive compounds present in cyanobacterial cells and not necessarily MC-LR may be responsible for negative effects observed during blooms. Hence, there is need for future studies to explore the potential toxic effects of bioactive compounds other than MCs in cyanobacterial crude extract.
Availability of data and materials
Raw data are available in the electronic supplementary information to the paper. Sources of animals and chemicals are provided in the text.
Code availability
R code is available from the authors on request.
References
Agathokleous E (2021) The rise and fall of photosynthesis: hormetic dose response in plants. J for Res 32:889–898
Agathokleous E, Kitao M, Harayama H, Calabrese EJ (2019) Temperature-induced hormesis in plants. J for Res 30:13–20
Alboukadel K, Marcin K, Przemyslaw B (2019) survminer: Drawing Survival Curves using 'ggplot2'. R package, 0.4.6. edn
Allen Y, Calow P, Baird DJ (1995) A mechanistic model of contaminant-induced feeding inhibition in Daphnia magna. Environ Toxicol Chem 14:1625–1630
Amorim CA, Moura A (2021) Ecological impacts of freshwater algal blooms on water quality, plankton biodiversity, structure, and ecosystem functioning. Sci Total Environ 758:143605. https://doi.org/10.1016/j.scitotenv.2020.143605
Babica BL, Maršalek B (2006) Exploring the natural role of microcystins—a review of effects on photoautotrophic organisms. J Phycol 42:9–20
Babica HK, Bártová K, Bláha L, Maršálek B (2007) Effects of dissolved microcystins on growth of planktonic photoautotrophs. Phycologia 46:137–142
Bittencourt-Oliveira MdC, Chia MA, de Oliveira HSB, Cordeiro Araújo MK, Molica RJR, Dias CTS (2015) Allelopathic interactions between microcystin-producing and non-microcystin-producing cyanobacteria and green microalgae: implications for microcystins production. J Appl Phycol 27:275–284. https://doi.org/10.1007/s10811-014-0326-2
Bolker BM (2008) Ecological models and data in R. Princeton University Press, Princeton
Bownik A (2016) Harmful algae: effects of cyanobacterial cyclic peptides on aquatic invertebrates-a short review. Toxicon 124:26–35. https://doi.org/10.1016/j.toxicon.2016.10.017
Briland RD, Stone JP, Manubolu M, Lee J, Ludsin S (2020) Cyanobacterial blooms modify food web structure and interactions in western Lake Erie. Harmful Algae 92:101586
Brooks BW, Lazorchak JM, Howard MD, Johnson M-VV, Morton SL, Perkins DA, Reavie ED, Scott GI, Smith SA, Steevens JA (2016) Are harmful algal blooms becoming the greatest inland water quality threat to public health and aquatic ecosystems? Environ Toxicol Chem 35:6–13
Brown JH, Gillooly JF, Allen AP, Savage VM, West G (2004) Toward a metabolic theory of ecology. Ecology 85:1771–1789
Buchwalter DB, Jenkins JJ, Curtis LR (2003) Temperature influences on water permeability and chlorpyrifos uptake in aquatic insects with differing respiratory strategies. Environ Toxicol Chem 22:2806–2812
Bui T, Dao T-S, Vo T-G, Lürling MJT (2018) Warming affects growth rates and microcystin production in tropical bloom-forming Microcystis strains. Toxins 10:123
Bui B-T, Wiegand C, Dinh KV, Dao T-S (2020) Responses of a tropical micro-crustacean, Daphnia lumholtzi, upon exposures to dissolved toxins and living cells of cyanobacteria. Environ Technol Innov 19:100973
Burkholder JM, Shumway SE, Glibert PM (2018) Food web and ecosystem impacts of harmful algae. Harmful algal blooms. Wiley, New york, pp 243–336
Calabrese E (2008) Hormesis: why it is important to toxicology and toxicologists. Environ Toxicol Chem 27:1451–1474
Carbonell JA, Stoks R (2020) Thermal evolution of life history and heat tolerance during range expansions toward warmer and cooler regions. Ecology 101:e03134
Chalifour A, Juneau P (2011) Temperature-dependent sensitivity of growth and photosynthesis of Scenedesmus obliquus, Navicula pelliculosa and two strains of Microcystis aeruginosa to the herbicide atrazine. Aquat Toxicol 103:9–17
Chalifour A, Arts MT, Kainz MJ, Juneau P (2014) Combined effect of temperature and bleaching herbicides on photosynthesis, pigment and fatty acid composition of Chlamydomonas reinhardtii. Eur J Phycol 49:508–515
Cham S (2012) Field guide to the larvae and exuviae of British dragonflies: dragonflies (Anisoptera) and damselflies (Zygoptera). British Dragonfly Society, Peterborough
Chen SL, Ou D, Gan N (2005) Chronic toxicity and responses of several important enzymes in Daphnia magna on exposure to sublethal microcystin-LR. Environ Toxicol 20:323–330
Chen XP, Li L, Xu J (2009) First identification of the hepatotoxic microcystins in the serum of a chronically exposed human population together with indication of hepatocellular damage. J Toxicol Sci 108:81–89
Chen DX, Shu T, Gulati RD, Liu Z (2013) Microcystins derived from lysing Microcystis cells do not cause negative effects on crustacean zooplankton in Lake Taihu, China. Aquat Ecol 47:379–387
Chen HuY, He J, Chen J, Giesy JP, Xie P (2017) Responses of the proteome and metabolome in livers of zebrafish exposed chronically to environmentally relevant concentrations of microcystin-LR. J Environ Sci Technol 51:596–607
Cheng C, Huang L, Ma R, Zhou Z, Diao J (2015) Enantioselective toxicity of lactofen and its metabolites in Scenedesmus obliquus. Algal Res 10:72–79
Chorus I, Welker M (2021) Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management. Taylor & Francis, London
Dao TS, Do-Hong L-C, Wiegand C (2010) Chronic effects of cyanobacterial toxins on Daphnia magna and their offspring. Toxicon 55:1244–1254
Dao T-S, Wiegand C, Bui B-T, Dinh KV (2018) Transgenerational effects of cyanobacterial toxins on a tropical micro-crustacean Daphnia lumholtzi across three generations. Environ Pollut 243:791–799
de Beeck LO, Verheyen J, Stoks R (2017) Integrating both interaction pathways between warming and pesticide exposure on upper thermal tolerance in high-and low-latitude populations of an aquatic insect. Environ Pollut 224:714–721
de Figueiredo DR, Azeiteiro UM, Esteves SM, Gonçalves FJ, Pereira MJ (2004) Microcystin-producing blooms—a serious global public health issue. Ecotoxicol Environ Saf 59:151–163
de Souza Machado AA, Zarfl C, Rehse S, Kloas W (2017) Low-dose effects: nonmonotonic responses for the toxicity of a Bacillus thuringiensis biocide to Daphnia magna. Environ Sci Technol 51:1679–1686
DeMott WR, Zhang QX, Carmichael WW (1991) Effects of toxic cyanobacteria and purified toxins on the survival and feeding of a copepod and three species of Daphnia. Limnol Oceanogr 36:1346–1357
Edwards R, Pascoe D (2018) Single species toxicity tests. Aquatic ecotoxicology. CRC Press, Boca Raton, pp 93–126
El-Sheekh M, Khairy H, El-Shenody R (2010) Allelopathic effects of cyanobacterium Microcystis aeruginosa Kützing on the growth and photosynthetic pigments of some algal species. Allelopath J 26:275–290
Erofeeva EA (2022) Environmental hormesis of non-specific and specific adaptive mechanisms in plants. Sci Total Environ 804:150059
Fang B et al (2018) Toxicity evaluation of 4, 4′-di-CDPS and 4, 4′-di-CDE on green algae Scenedesmus obliquus: growth inhibition, change in pigment content, and oxidative stress. Environ Sci Pollut Res 25:1–11
Figueredo CC, Giani A, Bird DF (2007) Does allelopathy contribute to Cylindrospermopsis raciborskii (Cyanobacteria) bloom occurrence and geographic expansion? J Phycol 43:256–265
Forbes VE (2000) Is hormesis an evolutionary expectation? Funct Ecol 14:12–24
Galic N, Grimm V, Forbes VE (2017) Impaired ecosystem process despite little effects on populations: modeling combined effects of warming and toxicants. Glob Change Biol 23:2973–2989. https://doi.org/10.1111/gcb.13581
Ger KA et al (2016) The interaction between cyanobacteria and zooplankton in a more eutrophic world. Harmful Algae 54:128–144. https://doi.org/10.1016/j.hal.2015.12.005
Ghadouani A, Pinel-Alloul B, Plath K, Codd GA, Lampert W (2004) Effects of Microcystis aeruginosa and purified microcystin-LR on the feeding behavior of Daphnia pulicaria. Limnol Oceanogr 49:666–679
Gomes MP, Juneau P (2017) Temperature and light modulation of herbicide toxicity on algal and cyanobacterial physiology. Front Environ Sci 5:50
Graham JL et al (2020) Cyanotoxin occurrence in large rivers of the United States. Inland Waters 10:109–117
Harke MJ et al (2016) A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 54:4–20
Henry Y, Piscart C, Charles S, Colinet H (2017) Combined effect of temperature and ammonia on molecular response and survival of the freshwater crustacean Gammarus pulex. Ecotoxicol Environ Saf 137:42–48
Heugens EH et al (2003) Temperature-dependent effects of cadmium on Daphnia magna: accumulation versus sensitivity. Environ Sci Technol 37:2145–2151
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363
Ibelings BW et al (2008) Ecosystem effects workgroup report. Cyanobacterial harmful algal blooms: state of the science and research needs. Springer, Berlin, pp 655–674
Janssen (2019) Cyanobacterial peptides beyond microcystins—a review on co-occurrence, toxicity, and challenges for risk assessment. Water Res 151:488–499
Jesus FT, Martins C, Nogueira AJ (2014) Changes in life-history parameters of Daphnia longispina (Cladocera, Crustacea) as a function of water chemistry. J Limnol. https://doi.org/10.4081/jlimnol.2014.886
Jos A et al (2005) Toxic cyanobacterial cells containing microcystins induce oxidative stress in exposed tilapia fish (Oreochromis sp.) under laboratory conditions. Aquat Toxicol 72:261–271
Jungmann BJ (1994) Toxicity to Daphnia of a compound extracted from laboratory and natural Microcystis spp., and the role of microcystins. Freshw Biol 32:13–20
Kenna D, Fincham WN, Dunn AM, Brown LE, Hassall C (2017) Antagonistic effects of biological invasion and environmental warming on detritus processing in freshwater ecosystems. Oecologia 183:875–886
Kim J, Seo JK, Yoon H, Kim PJ, Choi K (2014) Combined effects of the cyanobacterial toxin microcystin-LR and environmental factors on life-history traits of indigenous cladoceran Moina macrocopa. Environ Toxicol Chem 33:2560–2565
Klüttgen B, Dülmer U, Engels M, Ratte H (1994) ADaM, an artificial freshwater for the culture of zooplankton. Water Res 28:743–746
Kotak B, Zurawell R (2007) Cyanobacterial toxins in Canadian freshwaters: a review. Lake Reservoir Manage 23:109–122
Kozlowsky-Suzuki B, Wilson AE, da Silva F-F (2012) Biomagnification or biodilution of microcystins in aquatic foodwebs? Meta-analyses of laboratory and field studies. Harmful Algae 18:47–55
Lahti K, Rapala J, Färdig M, Niemelä M, Sivonen K (1997) Persistence of cyanobacterial hepatotoxin, microcystin-LR in particulate material and dissolved in lake water. Water Res 31:1005–1012
Lamb MC, Kimmel DG, Field EK (2019) The effects of temperature on Bosmina longirostris susceptibility to microcystin-LR acute toxicity. PLoS ONE 14:e0219342
Larras F, Lambert A-S, Pesce S, Rimet F, Bouchez A, Montuelle B (2013) The effect of temperature and a herbicide mixture on freshwater periphytic algae. Ecotoxicol Environ Saf 98:162–170
Li X, Ping X, Xiumei S, Zhenbin W, Liqiang X (2005) Toxicity of cypermethrin on growth, pigments, and superoxide dismutase of Scenedesmus obliquus. Ecotoxicol Environ Saf 60:188–192
Liang Y, Chen X, Lu X, Jin S, Min Y, Yang J (2017) Combined effects of microcystin and nitrite on the growth, lipid peroxidation, and antioxidant responses of the freshwater rotifer Brachionus calyciflorus. Aquat Toxicol 192:78–88
Liang Y, Lu X, Min Y, Liu L, Yang J (2018) Interactive effects of microcystin and ammonia on the reproductive performance and phenotypic traits of the rotifer Brachionus calyciflorus. Ecotoxicol Environ Saf 147:413–422
Loftin KA et al (2016) Cyanotoxins in inland lakes of the United States: Occurrence and potential recreational health risks in the EPA National Lakes Assessment 2007. Harmful Algae 56:77–90
Luo T, Chen J, Li X, Zhang S, Yao H, Peijnenburg WJ (2018) Effects of lomefloxacin on survival, growth and reproduction of Daphnia magna under simulated sunlight radiation. Ecotoxicol Environ Saf 166:63–70
Lürling M, van der Grinten E (2003) Life-history characteristics of Daphnia exposed to dissolved microcystin-LR and to the cyanobacterium Microcystis aeruginosa with and without microcystins. Environ Toxicol Chem 22:1281–1287
Ma H, Wu Y, Gan N, Zheng L, Li T, Song L (2015) Growth inhibitory effect of Microcystis on Aphanizomenon flos-aquae isolated from cyanobacteria bloom in Lake Dianchi, China. Harmful Algae 42:43–51
McKie BG, Schindler M, Gessner MO, Malmqvist B (2009) Placing biodiversity and ecosystem functioning in context: environmental perturbations and the effects of species richness in a stream field experiment. Oecologia 160:757–770
Moustaka-Gouni M, Sommer U (2020) Effects of harmful blooms of large-sized and colonial Cyanobacteria on aquatic food webs. Water 12:1587
Mowe MA, Mitrovic SM, Lim RP, Furey A, Yeo DC (2015) Tropical cyanobacterial blooms: a review of prevalence, problem taxa, toxins and influencing environmental factors. J Limnol. https://doi.org/10.4081/jlimnol.2014.1005
Müller MF, Colomer J, Serra T (2018) Temperature-driven response reversibility and short-term quasi-acclimation of Daphnia magna. PLoS ONE 13:e0209705
Nilsen E et al (2019) Critical review: grand challenges in assessing the adverse effects of contaminants of emerging concern on aquatic food webs. Environ Toxicol Chem 38:46–60
Noyes PD et al (2009) The toxicology of climate change: environmental contaminants in a warming world. Environ Int 35:971–986
OECD (2004) Test No. 202: Daphnia sp. acute immobilisation test. OECD Publishing, Berlin
OECD (2011) 201: Freshwater alga and cyanobacteria, growth inhibition test. OECD guidelines for the testing of chemicals, section 2
Oliver TH et al (2015) Biodiversity and resilience of ecosystem functions. Trends Ecol Evol 30:673–684
Omidi A, Esterhuizen-Londt M, Pflugmacher S (2018) Still challenging: the ecological function of the cyanobacterial toxin Microcystin—what we know so far. Toxin Reviews 37:87–105
Paerl HW (2008) Nutrient and other environmental controls of harmful cyanobacterial blooms along the freshwater–marine continuum. In: Hudnell HK (ed) Cyanobacterial harmful algal blooms: state of the science and research needs. Springer, New York, pp 217–237
Paerl HW, Huisman J (2008) Blooms like it hot. Science 320:57–58
Pflugmacher S (2002) Possible allelopathic effects of cyanotoxins, with reference to microcystin-LR, in aquatic ecosystems. Environ Toxicol 17:407–413
Pham T-L, Utsumi M (2018) An overview of the accumulation of microcystins in aquatic ecosystems. J Environ Manage 213:520–529
Pritchard DW, Paterson R, Bovy HC, Barrios-O’Neill D (2017) Frair: an R package for fitting and comparing consumer functional responses. Methods Ecol Evol 8:1528–1534
Pritchard D (2017) Frair: tools for Functional Response Analysis. R package, 0.5.100. edn
Rivetti C, Campos B, Barata C (2016) Low environmental levels of neuro-active pharmaceuticals alter phototactic behaviour and reproduction in Daphnia magna. Aquat Toxicol 170:289–296
Rogers D (1972) Random search and insect population models. J Anim Ecol 41:369–383
Rohrlack T, Dittmann E, Börner T, Christoffersen K (2001) Effects of cell-bound microcystins on survival and feeding of Daphnia spp. Appl Environ Microbiol 67:3523–3529
Sarnelle O, Gustafsson S, Hansson L-A (2010) Effects of cyanobacteria on fitness components of the herbivore Daphnia. J Plankton Res 32:471–477
Schulhof MA, Shurin JB, Declerck SAJ, Van de Waal DB (2019) Phytoplankton growth and stoichiometric responses to warming, nutrient addition and grazing depend on lake productivity and cell size. Glob Change Biol 25:2751–2762
Shahmohamadloo RS, Poirier DG, Almirall XO, Bhavsar SP, Sibley PK (2020a) Assessing the toxicity of cell-bound microcystins on freshwater pelagic and benthic invertebrates. Ecotoxicol Environ Saf 188:109945. https://doi.org/10.1016/j.ecoenv.2019.109945
Shahmohamadloo RS, Simmons DB, Sibley PK (2020b) Shotgun proteomics analysis reveals sub-lethal effects in Daphnia magna exposed to cell-bound microcystins produced by Microcystis aeruginosa. Comp Biochem Physiol d 33:100656
Sivonen K, Jones G (1999) Cyanobacterial toxins. In: Chorus I, Welker M (eds) Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management. CRC Press, Boca Raton (on behalf of the World Health Organization, Geneva, CH)
Skafi M et al (2021) Occurrence of microcystins, anabaenopeptins and other cyanotoxins in fish from a freshwater wildlife reserve impacted by harmful cyanobacterial blooms. Toxicon 194:44–52
Smutná M et al (2014) Acute, chronic and reproductive toxicity of complex cyanobacterial blooms in Daphnia magna and the role of microcystins. Toxicon 79:11–18
Stoks R, Geerts AN, De Meester L (2014) Evolutionary and plastic responses of freshwater invertebrates to climate change: realized patterns and future potential. Evol Appl 7:42–55
Sukenik A, Quesada A, Salmaso N (2015) Global expansion of toxic and non-toxic cyanobacteria: effect on ecosystem functioning. Biodivers Conserv 24:889–908
Šulčius S, Montvydienė D, Mazur-Marzec H, Kasperovičienė J, Rulevičius R, Cibulskaitė Ž (2017) The profound effect of harmful cyanobacterial blooms: From food-web and management perspectives. Sci Total Environ 609:1443–1450
Therneau TM, Grambsch PM (2000) Modeling survival data: extending the cox model. Springer, New York
Therneau T (2015) A Package for Survival Analysis in S. R package version 3.3–1, https://CRAN.R-project.org/package=survival
Thompson DJ (1978) Towards a realistic predator-prey model: the effect of temperature on the functional response and life history of larvae of the damselfly, Ischnura elegans. J Anim Ecol 47:757–767
UNDESA (2012) Back to our common future: sustainable development in the 21st Century (SD21) project. United Nations (UN), New York
Urrutia-Cordero P, Zhang H, Chaguaceda F, Geng H, Hansson LA (2020) Climate warming and heat waves alter harmful cyanobacterial blooms along the benthic-pelagic interface. Ecology 101:e03025
Van Dievel M, Stoks R, Janssens L (2017) Beneficial effects of a heat wave: higher growth and immune components driven by a higher food intake. J Exp Biol 220:3908–3915
Van Dinh K et al (2013) Susceptibility to a metal under global warming is shaped by thermal adaptation along a latitudinal gradient. Glob Change Biol 19:2625–2633
Verheyen J, Delnat V, Stoks R (2019) Increased daily temperature fluctuations overrule the ability of gradual thermal evolution to offset the increased pesticide toxicity under global warming. Environ Sci Technol 53:4600–4608
Villalobos-Jiménez G, Dunn AM, Hassall C (2017) Environmental noise reduces predation rate in an aquatic invertebrate. J Insect Conserv 21:839–847
Vinebrooke RD et al (2004) Impacts of multiple stressors on biodiversity and ecosystem functioning: the role of species co-tolerance. Oikos 104:451–457
Walls JT, Wyatt KH, Doll JC, Rubenstein EM, Rober AR (2018) Hot and toxic: temperature regulates microcystin release from cyanobacteria. Sci Total Environ 610:786–795
Wang L, Zi J, Xu R, Hilt S, Hou X, Chang X (2017) Allelopathic effects of Microcystis aeruginosa on green algae and a diatom: evidence from exudates addition and co-culturing. Harmful Algae 61:56–62. https://doi.org/10.1016/j.hal.2016.11.010
Wang YJ, Stoks R, Sentis A, Tüzün N (2020) Support for the climatic variability hypothesis depends on the type of thermal plasticity: lessons from predation rates. Oikos 129:1040–1050
Wei C, Zhang Y, Guo J, Han B, Yang X, Yuan J (2010) Effects of silica nanoparticles on growth and photosynthetic pigment contents of Scenedesmus obliquus. J Environ Sci 22:155–160
Wei H, Wang S, Xu EG, Liu J, Li X, Wang Z (2020) Synergistic toxicity of microcystin-LR and Cu to zebrafish (Danio rerio). Sci Total Environ 713:136393. https://doi.org/10.1016/j.scitotenv.2019.136393
Woodward G, Perkins DM, Brown LE (2010) Climate change and freshwater ecosystems: impacts across multiple levels of organization. Philos Trans R Soc B 365:2093–2106
Xiang X-L, Chen Y-Y, Xu Q-L, Zhu L-Y, Wen X-L, Xi Y-L (2017) Combined effects of temperature and the microcystin MC-LR on the feeding behavior of the rotifer Brachionus calyciflorus. Bull Environ Contam Toxicol 99:493–499
Zargar S, Krishnamurthi K, Saravana Devi S, Ghosh TK, Chakrabarti T (2006) Temperature-induced stress on growth and expression of Hsp in freshwater alga Scenedesmus quadricauda. Biomed Environ Sci 19:414–421
Zhang J, Xie Z, Wang Z (2016) Oxidative stress responses and toxin accumulation in the freshwater snail Radix swinhoei (Gastropoda, Pulmonata) exposed to microcystin-LR. Environ Sci Pollut Res 23:1353–1361
Zheng L, Xie P, Li Y, Yang H, Wang S, Guo N (2004) Variation of intracellular and extracellular microcystins in a shallow, hypereutrophic subtropical Chinese lake with dense cyanobacterial blooms. J Environ Contam Toxicol 73:698–706
Zhu R, Wang H, Shen H, Deng X, Chen J (2021) The dynamics and release characteristics of microcystins in the plateau Lake Erhai, Southwest China. Environ Sci Pollut Res Int 28:23473–23481
Zurawell RW, Chen H, Burke JM, Prepas EE (2005) Hepatotoxic cyanobacteria: a review of the biological importance of microcystins in freshwater environments. J Toxicol Environ Health Part B 8:1–37
Acknowledgements
The authors would like to thank the diligence and insight of the reviewers and editors of the manuscript, whose feedback considerably improved the writing of the paper and the interpretation of the results.
Funding
Funding was provided by the Tertiary Education Trust of Nigeria through a doctoral scholarship to OA.
Author information
Authors and Affiliations
Contributions
OA and CH originally formulated the idea with input from AMD and PK, OA and CH developed methodology, OA conducted fieldwork, APD established Microcystis cultures, OA and CH performed statistical analyses, and all the authors contributed to writing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by Ulrich Sommer.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Adekolurejo, O.A., Floyd, M., Dunn, A.M. et al. Combined effects of increased water temperature and cyanobacterial compounds exert heterogeneous effects on survival and ecological processes in key freshwater species. Oecologia 200, 515–528 (2022). https://doi.org/10.1007/s00442-022-05277-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-022-05277-7