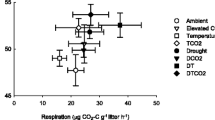
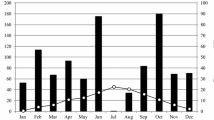
Abstract
Plant litter decomposition has been studied extensively in the context of both climate warming and increased atmospheric N deposition. However, much of this research is based on microbial responses, despite the potential for detritivores to contribute substantially to litter breakdown. We measured litter mass-loss responses to the combined effects of warming, N addition and detritivore access in a grass-dominated old field. We concurrently assessed the roles of litter treatment origin vs. microenvironment (direct warming and N-addition effects) to elucidate the mechanisms through which these factors affect decomposition. After 6 weeks, mass loss increased in N-addition plots, and it increased with detritivore access in the absence of warming. After 1 year, warming, N addition, and detritivore access all increased litter mass loss, although the effects of N addition and warming were non-additive in the detritivore-access plots. For the litter-origin experiment, mass loss after 6 weeks increased in litter from N-addition plots and warmed plots, but unlike the overall decomposition response, the N-addition effect was enhanced by detritivore access. Conversely, for the microenvironment experiment, detritivore access only increased mass loss in unfertilized plots. After 1 year, detritivore access increased mass loss in the litter-origin and microenvironment experiments, but there were no warming or N-addition effects. Overall, our results provide support for a substantial role of detritivores in promoting litter mass loss in our system. Moreover, they reveal important interactions between litter origin, microclimate and detritivores in determining decomposition responses to global change.




Similar content being viewed by others
References
A’Bear AD, Boddy L, Jones TH (2012) Impacts of elevated temperature on the growth and functioning of decomposer fungi are influenced by grazing collembola. Glob Change Biol 18:1823–1832
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449
Aerts R (2006) The freezer defrosting: global warming and litter decomposition rates in cold biomes. J Ecol 94:713–724
Allison SD, Treseder KK (2008) Warming and drying suppress microbial activity and carbon cycling in boreal forest soils. Glob Change Biol 14:2898–2909
Allison SD, Wallenstein MD, Bradford MA (2010) Soil-carbon response to warming dependent on microbial physiology. Nat Geosci 3:336–340
An Y, Wan S, Zhou X, Subedar AA, Wallace LL, Luo Y (2005) Plant nitrogen concentration, use efficiency, and contents in a tallgrass prairie ecosystem under experimental warming. Glob Change Biol 11:1733–1744
Ayres E, Stelzer H, Berg S, Wall DH (2009a) Soil biota accelerate decomposition in high-elevation forests by specializing in the breakdown of litter produced by the plant species above them. J Ecol 97:901–912
Ayres E, Stelzer H, Simmons BL, Simpson RT, Steinweg JM, Wallenstein MD, Mellor N, Parton WJ, Moore JC, Wall DH (2009b) Home field advantage accelerates leaf litter decomposition in forests. Soil Biol Biogeochem 41:606–610
Balser TC, McMahon KD, Bart D, Bronson D, Coyle DR, Craig N, Flores-Mangual ML, Forshay K, Jones SE, Kent AE, Shade AL (2006) Bridging the gap between micro- and macro-scale perspectives on the role of microbial communities in global change ecology. Plant Soil 289:59–70
Bastow JL (2011) Resource quality in a soil food web. Biol Fertil Soil 48:501–510
Beatty SW, Sholes ODV (1988) Leaf litter effect on plant-species composition of deciduous forest treefall traps. Can J For Res 18:553–559
Bell TH, Klironomos JN, Henry HAL (2010) Seasonal responses of extracellular enzyme activity and microbial biomass to warming and nitrogen addition. Soil Sci Soc Am J 74:820–828
Blankinship JC, Niklaus PA, Hungate BA (2011) A meta-analysis of responses of soil biota to global change. Oecologia 165:553–565
Bokhorst S, Wardle D (2013) Microclimate within litter bags of different mesh size: implications for the “arthropod effect” on litter decomposition. Soil Biol Biogeochem 58:147–152
Bosy JL, Reader RJ (1995) Mechanisms underlying the suppression of forb seedling emergence by grass (Poa pratensis) litter. Funct Ecol 9:635–639
Bradford M, Tordoff G, Eggers T, Jones T, Newington J (2002) Microbiota, fauna, and mesh size interactions in litter decomposition. Oikos 99:317–323
Bradford MA, Davies CA, Frey SD, Maddox TR, Melillo JM, Mohan JE, Reynolds JF, Treseder KK, Wallenstein MD (2008) Thermal adaptation of soil microbial respiration to elevated temperature. Ecol Lett 11:1316–1327
Briones MJI, Ineson P, Piearce TG (1997) Effects of climate change on soil fauna; responses of enchytraeids, Diptera larvae and tardigrades in a transplant experiment. Appl Soil Ecol 6:117–134
Brzostek ER, Blair JM, Dukes JS, Frey SD, Hobbie SE, Melillo JM, Mitchell RJ, Pendall E, Reich PB, Shaver GR, Stefanski A, Tjoelker MG, Finzi AC (2012) The effect of experimental warming and precipitation change on proteolytic enzyme activity: positive feedbacks to nitrogen availability are not universal. Glob Change Biol 18:2617–2625
Butenschoen O, Scheu S, Eisenhauer N (2011) Interactive effects of warming, soil humidity and plant diversity on litter decomposition and microbial activity. Soil Biol Biochem 43:1902–1907
Carreiro MM, Sinsabaugh RL, Repert DA, Parkhurst DF (2000) Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 81:2359–2365
Chapin FI, Matson P, Mooney H (2002) Principles of terrestrial ecosystem ecology. Springer, New York
Conant RT, Drijber RA, Haddix ML, Parton WJ, Paul EA, Plante AF, Six J, Steinweg JM (2008) Sensitivity of organic matter decomposition to warming varies with its quality. Glob Change Biol 14:868–877
Coûteaux MM, Mousseau M, Celerier ML, Bottner P (1991) Increased atmospheric CO2 and litter quality—decomposition of sweet chestnut leaf litter with animal food webs of different complexities. Oikos 61:54–64
Coûteaux MM, Bottner P, Berg B (1995) Litter decomposition, climate and litter quality. Trends Ecol Evol 10:63–66
Dangles O, Herrera M, Mazoyer C, Silvain J-F (2013) Temperature-dependent shifts in herbivore performance and interactions drive nonlinear changes in crop damages. Glob Change Biol 19:1056–1063
Deutsch ES, Bork EW, Willms WD (2010) Soil moisture and plant growth responses to litter and defoliation impacts in Parkland grasslands. Agric Ecosyst Environ 135:1–9
Fog K (1988) The effect of added nitrogen on the rate of decomposition of organic matter. Biol Rev 63:433–462
Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, Asner GP, Cleveland CC, Green PA, Holland EA, Karl DM, Michaels AF, Porter JH, Townsend AR, Vorosmarty CJ (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 70:153–226
Gan HJ, Zak DR, Hunter MD (2013) Chronic nitrogen deposition alters the structure and function of detrital food webs in a northern hardwood ecosystem. Ecol Appl 23:1311–1321
García-Palacios P, Maestre FT, Kattge J, Wall DH (2013) Climate and litter quality differently modulate the effects of soil fauna on litter decomposition across biomes. Ecol Lett 16:1045–1053
Hagerty TP, Kingston MS (2011) The soils of Middlesex County. Ontario Ministry of Agriculture and Food, Guelph
Henry HAL, Cleland EE, Field CB, Vitousek PM (2005) Interactive effects of elevated CO2, N deposition and climate change on plant litter quality in a California annual grassland. Oecologia 142:465–473
Henry HAL, Hutchison JS, Kim M, McWhirter BD (2014) Context matters for warming: interannual variation in grass biomass responses to seven years of warming and N addition. Ecosystems (in press)
Hobbie SE (2005) Contrasting effects of substrate and fertilizer nitrogen on the early stages of litter decomposition. Ecosystems 8:644–656
Hobbie SE (2008) Nitrogen effects on decomposition: a five-year experiment in eight temperate sites. Ecology 89:2633–2644
Holman JD, Hunt C, Thill D (2007) Structural composition, growth stage, and cultivar effects on Kentucky bluegrass forage yield and nutrient composition. Agronomy 99:195–202
Holmstrup M, Sørensen JG, Maraldo K, Schmidt IK, Mason S, Tietema A, Smith AR, Emmett B, Schmelz RM, Bataillon T, Beier C, Ehlers BK (2012) Increased frequency of drought reduces species richness of enchytraeid communities in both wet and dry heathland soils. Soil Biol Biochem 53:43–49
Hunt HW, Ingham ER, Coleman DC, Elliott ET, Reid CPP (1988) Nitrogen limitation of production and decomposition in prairie, mountain meadow, and pine forest. Ecology 69:1009–1016
Hutchison JS, Henry HAL (2010) Additive effects of warming and increased nitrogen deposition in a temperate old field: plant productivity and the importance of winter. Ecosystems 13:661–672
Johnson DW (1992) Nitrogen retention in forest soils. J Environ Qual 21:1–12
Kampichler C, Bruckner A (2009) The role of microarthropods in terrestrial decomposition: a meta-analysis of 40 years of litterbag studies. Biol Rev 84:375–389
Kardol P, Reynolds WN, Norby RJ, Classen AT (2011) Climate change effects on soil microarthropod abundance and community structure. Appl Soil Ecol 47:37–44
Knorr M, Frey SD, Curtis PS (2005) Nitrogen additions and litter decomposition: a meta-analysis. Ecology 86:3252–3257
Levings SC, Windsor DM (1996) Seasonal and annual variation in litter arthropod populations. In: Leigh EG Jr, Rand AS, Windsor DM (eds) The ecology of a tropical forest: seasonal rhythms and long-term changes. Smithsonian Institutional Press, Washington, DC, pp 355–387
Lohm U, Lundkvist H, Persson T, Wiren A (1977) Effects of nitrogen fertilization on the abundance of enchytraeids and microarthropods in Scots pine forests. Stud For Suec 140:1–23
McGill WB, Cole CV (1981) Comparative aspects of cycling of organic C, N, S and P through soil organic matter. Geoderma 26:267–286
Melick DR, Seppelt RD (2004) Loss of soluble carbohydrates and changes in freezing point of Antarctic bryophytes after leaching and repeated free-thaw cycles. Antarct Sci 4:399–404
O’Lear H, Blair J (1999) Responses of soil microarthropods to changes in soil water availability in tallgrass prairie. Biol Fertil Soil 29:207–217
Pendall E, Rustad L, Schimel J (2008) Towards a predictive understanding of belowground process responses to climate change: have we moved any closer? Funct Ecol 22:937–940
Peñuelas J, Sardans J, Ogaya R, Estiarte M (2008) Nutrient stoichiometric relations and biogeochemical niche in coexisting plant species: effect of simulated climate change. Pol J Ecol 56:613–622
Pritchard SG (2011) Soil organisms and global climate change. Plant Pathol 60:82–99
Rouifed S, Handa IT, David J-F, Hättenschwiler S (2010) The importance of biotic factors in predicting global change effects on decomposition of temperate forest leaf litter. Oecologia 163:247–256
Seastedt TR (1984) The role of microarthropods in decomposition and mineralization processes. Annu Rev Entomol 29:25–46
Shen KP, Harte J (2000) Ecosystem climate manipulations. In: Sala OE, Jackson RB, Mooney HA, Howarth RW (eds) Methods in ecosystem science. Springer, New York, pp 353–369
Singh JS, Gupta SR (1977) Plant decomposition and soil respiration in terrestrial ecosystems. Bot Rev 43:449–528
Sjursen H, Michelsen A, Jonasson S (2005) Effects of long-term soil warming and fertilisation on microarthropod abundances in three sub-arctic ecosystems. Appl Soil Ecol 30:148–161
Slade E, Riutta T (2012) Interacting effects of leaf litter species and macrofauna on decomposition in different litter environments. Basic Appl Ecol 13:423–431
Suffling R, Smith DW (1974) Litter decomposition studies using mesh bags: spillage inaccuracies and the effects of repeated artificial drying. Can J Bot 52:2157–2163
Swift MJ, Heal OW, Anderson JM (1979) Decomposition in Terrestrial Ecosystems. University of California Press, Berkeley
Taylor BR, Parkinson D (1988) Does repeated freezing and thawing accelerate decay of leaf litter? Soil Biology Biogeochem 20:657–665
Templer PH, Reinmann AB (2011) Multi-factor global change experiments: what have we learned about terrestrial carbon storage and exchange? New Phytol 192:797–800
Tian G, Brussaard L, Kang BT (1993) Biological effects of plant residues with contrasting chemical compositions under humd tropical conditions: effects on soil fauna. Soil Biol Biochem 25:731–737
Tian G, Adejuyigbe CO, Adeoye GO, Kang BT (1998) Role of soil microarthropods in leaf decomposition and N release under various land-use practices in the humid tropics. Pedobiologia 42:33–42
Turner MM, Henry HAL (2009) Interactive effects of warming and increased nitrogen deposition on 15-N tracer retention in a temperate old field: seasonal trends. Glob Change Biol 15:2885–2893
Vitousek PM (1982) Nutrient cycling and nutrient use efficiency. Am Nat 119:553–572
Wall DH, Bradford MA, St. John MG, Trofymow JA, Behan-Pelletier V, Bignell DE, Dangerfield JM, Parton WJ, Rusek J, Voigt W, Wolters V, Gardel HZ, Ayuke FO, Bashford R, Beljakova OI, Bohlen PJ, Brauman A, Flemming S, Henschel JR, Johnson DL, Jones TH, Kovarova M, Kranabetter JM, Kutny L, Lin K-C, Maryati M, Masse D, Pokarzhevskii A, Rahman H, Sabar MG, Salamon J-A, Swift MJ, Varela A, Vasconcelos HL, White D, Zou X (2008) Global decomposition experiment shows soil animal impacts on decomposition are climate-dependent. Glob Change Biol 14:2661–2677
Wan S, Norby RJ, Ledford J, Weltzin JF (2007) Responses of soil respiration to elevated CO2, air warming, and changing soil water availability in a model old-field grassland. Glob Change Biol 13:2411–2424
Wei CZ, Zheng HF, Li Q, Lu XT, Yu Q, Zhang HY, Chen QS, He NP, Kardol P, Liang WJ, Han XG (2012) Nitrogen addition regulates soil nematode community composition through ammonium suppression. PLoS ONE 7:e43384
Wickings K, Grandy AS (2013) Management intensity interacts with litter chemistry and climate to drive temporal patterns in arthropod communities during decomposition. Pedobiologia 56:105–112
Wolters V (2000) Invertebrate control of soil organic matter stability. Biol Fertil Soils 31:1–19
Wu H, Lu X, Jiang M, Bao X (2009) Impacts of soil fauna on litter decomposition at different succession stages of wetland in Sanjiang Plain, China. Chin Geog Sci 19:258–264
Xin W, Yin X, Song B (2012) Contributions of soil fauna to litter decomposition in Songnen sandy lands in Northeastern China. J Arid Environ 77:90–95
Xu G-L, Kuster TM, Günthardt-Goerg MS, Dobbertin M, Li M-H (2012a) Seasonal exposure to drought and air warming affects soil collembola and mites. PLoS ONE 7:e43102
Xu ZF, Pu XZ, Yin HJ, Zhao CZ, Liu Q, Wu FZ (2012b) Warming effects on the early decomposition of three litter types, Eastern Tibetan Plateau, China. Eur J Soil Sci 63:360–367
Zhu X, Gao B, Yuan S, Hu Y (2010) Community structure and seasonal variation of soil arthropods in the forest-steppe ecotone of the mountainous region in Northern Hebei, China. J Mt Sci 7:187–196
Acknowledgments
The infrastructure for this experiment was funded by the Canadian Foundation for Innovation and the Ontario Research Fund. This work was also supported by a Natural Sciences and Engineering Research Council of Canada Discovery Grant to HALH and an NSERC Post-Graduate Scholarship to ERDM.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Russell Monson.
Rights and permissions
About this article
Cite this article
Moise, E.R.D., Henry, H.A.L. Interactive responses of grass litter decomposition to warming, nitrogen addition and detritivore access in a temperate old field. Oecologia 176, 1151–1160 (2014). https://doi.org/10.1007/s00442-014-3068-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-014-3068-6