Abstract
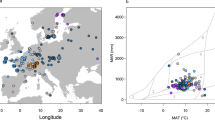
Species may respond in three ways to environmental change: adapt, migrate, or go extinct. Studies of latitudinal clines can provide information on whether species have adapted to abiotic stress such as temperature and drought in the past and what the traits underlying adaptation are. We investigated latitudinal trait variation and response to drought in North American populations of Arabidopsis lyrata. Plants from nine populations collected over 13° latitude were grown under well-watered and dry conditions. A total of 1,620 seedlings were raised and 12 phenological, physiological, morphological, and life history traits were measured. Two traits, asymptotic rosette size and the propensity to flower, were significantly associated with latitude: plants from northern locations grew to a larger size and were more likely to flower in the first season. Most traits displayed a plastic response to drought, but plasticity was never related linearly with latitude nor was it enhanced in populations from extreme latitudes with reduced water availability. Populations responded to drought by adopting mixed strategies of resistance, tolerance, and escape. The study shows that latitudinal adaptation in A. lyrata involves the classic life history traits, size at and timing of reproduction. Contrary to recent theoretical predictions, adaptation to margins is based on fixed trait differences and not on phenotypic plasticity, at least with respect to drought.




Similar content being viewed by others
References
Al-Shehbaz IA (2010) Magnoliophyta: Salicaceae to Brassicaceae. In: Flora of North America Editorial Committee (eds) Flora of North America north of Mexico. Oxford University Press, New York
Bell G, Collins S (2008) Adaptation, extinction and global change. Evol Appl 1:3–16
Bridle JR, Vines TH (2007) Limits to evolution at range margins: when and why does adaptation fail? Trends Ecol Evol 22:140–147
Chevin LM, Lande R (2011) Adaptation to marginal habitats by evolution of increased phenotypic plasticity. J Evol Biol 24:1462–1476
Conover DO, Schultz ET (1995) Phenotypic similarity and the evolutionary significance of countergradient variation. Trends Ecol Evol 10:248–251
Conover DO, Duffy TA, Hice LA (2009) The covariance between genetic and environmental influences across ecological gradients. Ann NY Acad Sci 1168:100–129
Cornelissen JHC, Lavorel S, Garnier E, Díaz S, Buchmann N, Gurvich DE, Reich PB, ter Steege H, Morgan HD, van der Heijden MGA, Pausas JG, Poorter H (2003) A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust J Bot 51:335–380
Davis MB, Shaw RG (2001) Range shifts and adaptive responses to quaternary climate change. Science 292:673–679
De Frenne P, Brunet J, Shevtsova A, Kolb A, Graae BJ, Chabrerie O, Cousins SAO, Decocq G, De Schrijver A, Diekmann M, Gruwez R, Heinken T, Hermy M, Nilsson C, Stanton S, Tack W, Willaert J, Verheyen K (2011) Temperature effects on forest herbs assessed by warming and transplant experiments along a latitudinal gradient. Glob Change Biol 17:3240–3253
Edwards KR, Bastlová D, Edwards-Jonášová M, Kvĕt J (2011) A comparison of univariate and multivariate methods for analyzing clinal variation in an invasive species. Hydrobiologia 674:119–131
El-Keblawy A, Lovett-Doust J (1999) Maternal effects in the progeny generation in zucchini, Cucurbita pepo (Cucurbitaceae). Int J Plant Sci 160:331–339
Etterson JR (2004) Evolutionary potential of Chamaecrista fasciculata in relation to climate change. I. Clinal patterns of selection along an environmental gradient in the Great Plains. Evolution 58:1446–1458
Farquhar GD, Richards RA (1984) Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Aust J Plant Physiol 11:539–552
Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40:503–537
Franks SJ, Weis AE (2008) A change in climate causes rapid evolution of multiple life-history traits and their interactions in an annual plant. J Evol Biol 21:1321–1334
Grant PR, Grant BR (2002) Unpredictable evolution in a 30-year study of Darwin’s finches. Science 296:707–711
Griffin PC, Willi Y (2014) Evolutionary shifts to self-fertilization restricted to geographic range margins in North American Arabidopsis lyrata. Ecol Lett 17:484–490
Griffith TM, Watson MA (2005) Stress avoidance in a common annual: reproductive timing is important for local adaptation and geographic distribution. J Evol Biol 18:1601–1612
Hetherington AM, Woodward FI (2003) The role of stomata in sensing and driving environmental change. Nature 424:901–908
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Hill WG, Rasbash J (1986a) Models of long term artificial selection in finite population. Genet Res 48:41–50
Hill WG, Rasbash J (1986b) Models of long-term artificial selection in finite population with recurrent mutation. Genet Res 48:125–131
Hoffmann AA, Willi Y (2008) Detecting genetic responses to environmental change. Nat Rev Genet 9:421–432
Jenkins DG, Carey M, Czerniewska J, Fletcher J, Hether T et al (2010) A meta-analysis of isolation by distance: relic or reference standard for landscape genetics? Ecography 33:315–320
Jump AS, Peñuelas J (2005) Running to stand still: adaptation and the response of plants to rapid climate change. Ecol Lett 8:1010–1020
Kawecki TJ (2008) Adaptation to marginal habitats. Annu Rev Ecol Evol Syst 39:321–342
Kincaid DT, Schneider RB (1983) Quantification of leaf shape with a microcomputer and Fourier transform. Can J Bot 61:2333–2342
Knight CA, Vogel H, Kroymann J, Shumate A, Witsenboer H, Mitchell-Olds T (2006) Expression profiling and local adaptation of Boechera holboellii populations for water use efficiency across a naturally occurring water stress gradient. Mol Ecol 15:1229–1237
Ludlow MM (1989) Strategies of response to water stress. In: Kreeb KH, Richter H, Hinckley TM (eds) Structural and functional responses to environmental stresses: water shortage. SPB, the Hague, pp 269–281
Lynch M, Lande R (1993) Evolution and extinction in response to environmental change. In: Kareiva PM, Kingsolver JG, Huey RB (eds) Biotic interactions and global change. Sinauer, Sunderland, pp 234–250
Maherali H, Reid CD, Polley HW, Johnson HB, Jackson RB (2002) Stomatal acclimation over a subambient to elevated CO2 gradient in a C3/C4 grassland. Plant Cell Environ 25:557–566
Maron JL, Vilà M, Bommarco R, Elmendorf S, Beardsley P (2004) Rapid evolution of an invasive plant. Ecol Monogr 74:261–280
Maron JL, Elmendorf SC, Vilà M (2007) Contrasting plant physiological adaptation to climate in the native and introduced range of Hypericum perforatum. Evolution 61:1912–1924
McKay JK, Richards JH, Mitchell-Olds T (2003) Genetics of drought adaptation in Arabidopsis thaliana. 1. Pleiotropy contributes to genetic correlations among ecological traits. Mol Ecol 12:1137–1151
Neuffer B (2011) Native range variation in Capsella bursa-pastoris (Brassicaceae) along a 2500 km latitudinal transect. Flora 206:107–119
Nicotra AB, Leigh A, Boyce CK, Jones CS, Niklas KJ, Royer DL, Tsukaya H (2011) The evolution and functional significance of leaf shape in the angiosperms. Funct Plant Biol 38:535–552
Novy A, Flory SL, Hartman JM (2013) Evidence for rapid evolution of phenology in an invasive grass. J Evol Biol 26:443–450
Paccard A, Vance M, Willi Y (2013) Weak impact of fine-scale landscape heterogeneity on evolutionary potential in Arabidopsis lyrata. J Evol Biol 26:2331–2340
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37:637–669
Pickett STA (1989) Space-for-time substitution as an alternative to long-term studies. In: Likens GE (ed) Long-term studies in ecology: approaches and alternatives. Springer, New York Berlin Heidelberg, pp 110–135
Picotte JJ, Rhode JM, Cruzan MB (2009) Leaf morphological responses to variation in water availability for plants in the Piriqueta caroliniana complex. Plant Ecol 200:267–275
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rasband WS (2011) ImageJ. US National Institutes of Health, Bethesda, MD. http://imagej.nih.gov/ij/
Reich PB, Walters MB, Ellsworth DS (1997) From tropics to tundra: global convergence in plant functioning. Proc Natl Acad Sci USA 94:13730–13734
Riihimäki M, Podolsky R, Kuittinen H, Koelewijn H, Savolainen O (2005) Studying genetics of adaptive variation in model organisms: flowering time variation in Arabidopsis lyrata. Genetica 123:63–74
Ritz C, Streibig JC (2005) Bioassay analysis using R. J Stat Softw 12:1–22
Robertson A (1960) A theory of limits in artificial selection. Proc R Soc Lond B 153:234–249
SAS Institute (2002) SAS: version 9.2. SAS Institute, Cary, NC
Schmickl R, Jørgensen MH, Brysting AK, Koch MA (2010) The evolutionary history of the Arabidopsis lyrata complex: a hybrid in the amphi-Beringian area closes a large distribution gap and builds up a genetic barrier. BMC Evol Biol 10:98
Sexton JP, McIntyre PJ, Angert AL, Rice KJ (2009) Evolution and ecology of species range limits. Annu Rev Ecol Evol Syst 40:415–436
Singer JD (1998) Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. J Educ Behav Stat 24:323–355
Sletvold N, Ågren J (2012) Variation in tolerance to drought among Scandinavian populations of Arabidopsis lyrata. Evol Ecol 26:559–577
Steets JA, Takebayashi N, Byrnes JM, Wolf DE (2010) Heterogeneous selection on trichome production in Alaskan Arabidopsis kamchatica (Brassicaceae). Am J Bot 97:1098–1108
Stinchcombe JR, Weinig C, Ungerer M, Olsen KM, Mays C, Halldorsdottir SS, Purugganan MD, Schmitt J (2004) A latitudinal cline in flowering time in Arabidopsis thaliana modulated by the flowering time gene FRIGIDA. Proc Natl Acad Sci USA 101:4712–4717
Trabucco A, Zomer RJ (2010) Global soil water balance geospatial database. CGIAR Consortium for Spatial Information. Available at: http://www.cgiar-csi.org
Travis SE, Grace JB (2010) Predicting performance for ecological restoration: a case study using Spartina alterniflora. Ecol Appl 20:192–204
Vekemans X, Hardy OJ (2004) New insights from fine-scale spatial genetic structure analyses in plant populations. Mol Ecol 13:921–935
Wei M, Caballero A, Hill WG (1996) Selection response in finite populations. Genetics 144:1961–1974
Willi Y, Hoffmann AA (2009) Demographic factors and genetic variation influence population persistence under environmental change. J Evol Biol 22:124–133
Willi Y, Määttänen K (2010) Evolutionary dynamics of mating system shifts in Arabidopsis lyrata. J Evol Biol 23:2123–2131
Willi Y, Määttänen K (2011) The relative importance of factors determining genetic drift: mating system, spatial genetic structure, habitat and census size in Arabidopsis lyrata. New Phytol 189:1200–1209
Woods EC, Hastings AP, Turley NE, Heard SB, Agrawal AA (2012) Adaptive geographical clines in the growth and defense of a native plant. Ecol Monogr 82:149–168
Wu CA, Lowry DB, Nutter LI, Willis JH (2010) Natural variation for drought-response traits in the Mimulus guttatus species complex. Oecologia 162:23–33
Acknowledgments
Collection permits were granted by the US Army at Fort Leonard Wood, the Iowa State Preserves Advisory Board, the Iowa Department of Natural Resources, the Virginia Department of Conservation and Recreation, the Nature Conservancy of Maryland, the US National Park Service, and the New York State Office of Parks. Seeds at Fort Leonard Wood were kindly sampled by Joe Proffitt. We would like to thank the many people who helped with measuring plants, Olivier Bachmann, Emmanuel Bonjour, Benjamin Dauphin, Philippa Griffin, Adnan Peco, Marta Anda Perez, Katia Presani, Anouk Sarr, Reyhan Sonmez, and Julien Vieu. Josh Van Buskirk provided comments on the manuscript. Mass spectrometry was performed at the University of New Hampshire Stable Isotope Laboratory, Durham, NH, USA, and the Cornell University Stable Isotope Laboratory, Ithaca, NY, USA. The research was supported by the Swiss National Science Foundation (PP00P3-123396/1) and the Fondation Pierre Mercier pour la Science. The experiments were performed in Switzerland and comply with the current laws of this country.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Christina Marie Caruso.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Paccard, A., Fruleux, A. & Willi, Y. Latitudinal trait variation and responses to drought in Arabidopsis lyrata . Oecologia 175, 577–587 (2014). https://doi.org/10.1007/s00442-014-2932-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-014-2932-8