Abstract
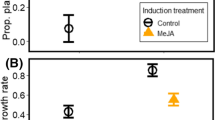
Plants are frequently attacked by both pathogens and insects, and an attack from one can induce plant responses that affect resistance to the other. However, we currently lack a predictive framework for understanding how pathogens, their vectors, and other herbivores interact. To address this gap, we have investigated the effects of a viral infection in the host plant on both its aphid vector and non-vector herbivores. We tested whether the infection by three different strains of Potato virus Y (PVYNTN, PVYNO and PVYO) on tomato plants affected: (1) the induced plant defense pathways; (2) the abundance and fecundity of the aphid vector (Macrosiphum euphorbiae); and (3) the performance of two non-vector species: a caterpillar (Trichoplusia ni) and a beetle (Leptinotarsa decemlineata). While infection by all three strains of PVY induced the salicylate pathway, PVYNTN induced a stronger and longer response. Fecundity and density of aphids increased on all PVY-infected plants, suggesting that the aphid response is not negatively associated with salicylate induction. In contrast, the performance of non-vector herbivores positively correlated with the strength of salicylate induction. PVYNTN infection decreased plant resistance to both non-vector herbivores, increasing their growth rates. We also demonstrated that the impact of host plant viral infection on the caterpillar results from host plant responses and not the effects of aphid vector feeding. We propose that pathogens chemically mediate insect–plant interactions by activating the salicylate pathway and decreasing plant resistance to chewing insects, which has implications for both disease transmission and insect community structure.




Similar content being viewed by others
References
Abe H et al (2012) Antagonistic plant defense system regulated by phytohormones assists interactions among vector insect, thrips and a tospovirus. Plant Cell Physiol 53:204–212
Alvarez AE, Garzo E, Verbeek M, Vosman B, Dicke M, Tjallingii WF (2007) Infection of potato plants with Potato leafroll virus changes attraction and feeding behaviour of Myzus persicae. Entomol Exp Appl 125:135–144
Asselbergh B, Achuo A, Hofte M, Van Gijsegem F (2008) Abscisic acid deficiency leads to rapid activation of tomato defence responses upon infection with Erwinia chrysanthemi. Mol Plant Pathol 9:11–24
Baebler Š, Krečič-Stres H, Rotter A, Kogovšek P, Cankar K, Kok EJ, Gruden K, Kovač M, Zel J, Pompe-Novak M, Ravnikar M (2009) PVYNTN elicits a diverse gene expression response in different potato genotypes in the first 12 h after inoculation. Mol Plant Pathol 10:263–275
Belliure B, Janssen A, Maris PC, Peter D, Sabelis MW (2005) Herbivore arthropods benefit from vectoring plant viruses. Ecol Lett 8:70–79
Belliure B, Janssen A, Sabelis MW (2008) Herbivore benefits from vectoring plant virus through reduction of period of vulnerability to predation. Oecologia 156:797–806
Belliure B, Sabelis MW, Janssen A (2010) Vector and virus induce plant responses that benefit a non-vector herbivore. Basic Appl Ecol 11:162–169
Blua MJ, Perring TM, Madore MA (1994) Plant virus-induced changes in aphid population development and temporal fluctuations in plant nutrients. J Chem Ecol 20:691–707
Boquel S, Ameline A, Giordanengo P (2011a) Assessing aphids potato virus Y-transmission efficiency: a new approach. J Virol Methods 178:63–67
Boquel S, Giordanengo P, Ameline A (2011b) Divergent effects of PVY-infected potato plant on aphids. Eur J Plant Pathol 129:507–510
Cardoza YJ, Lait CG, Schmelz EA, Huang J, Tumlinson JH (2003) Fungus-induced biochemical changes in peanut plants and their effect on development of beet armyworm, Spodoptera exigua Hubner (Lepidoptera : Noctuidae) larvae. Environ Entomol 32:220–228
Castle SJ, Berger PH (1993) Rates of growth and increase of Myzus persicae on virus-infected potatoes according to type of virus-vector relationship. Entomol Exp Appl 69:51–60
Colvin J et al (2006) Host-plant viral infection effects on arthropod-vector population growth, development and behaviour: management and epidemiological implications. Adv Virus Res 67:419–452
Cui JP, Jander G, Racki LR, Kim PD, Pierce NE, Ausubel FM (2002) Signals involved in Arabidopsis resistance to Trichoplusia ni caterpillars induced by virulent and avirulent strains of the phytopathogen Pseudomonas syringae. Plant Physiol 129:551–564
Cui J et al (2005) Pseudomonas syringae manipulates systemic plant defenses against pathogens and herbivores. Proc Natl Acad Sci USA 102:1791–1796
Delaney TP (1994) A central role of salicylic-acid in plant-disease resistance. Science 266:1793
Eigenbrode SD, Ding H, Shiel P, Berger PH (2002) Volatiles from potato plants infected with Potato leafroll virus attract and arrest the virus vector, Myzus persicae (Homoptera; Aphididae). Proc Natl Acad Sci USA 269:455–460
Felton GW et al (1999) Inverse relationship between systemic resistance of plants to microorganisms and to insect herbivory. Curr Biol 9:317–320
Fidantsef AL, Stout MJ, Thaler JS, Duffey SS, Bostock RM (1999) Signal interactions in pathogen and insect attack: expression of lipoxygenase, proteinase inhibitor II, and pathogenesis-related protein P4 in the tomato, Lycopersicon esculentum. Physiol Mol Plant Pathol 54:97–114
Goggin FL, Williamson VM, Ullman DE (2001) Variability in the response of Macrosiphum euphorbiae and Myzus persicae (Hemiptera: Aphididae) to the tomato resistance gene Mi. Environ Entomol 30:101–106
Hare JD, Dodds JA (1987) Survival of the Colorado potato beetle on virus-infected tomato in relation to plant nitrogen and alkaloid content. Entomol Exp Appl 44:31–35
Harrington R, Gibson RW (1989) Transmission of Potato virus Y by aphids trapped in potato crops in southern England. Potato Res 32:167–174
Herbers K, Takahata Y, Melzer M, Mock H-P, Hajirezaei M, Sonnewald U (2000) Regulation of carbohydrate partitioning during the interaction of Potato virus Y with tobacco. Mol Plant Pathol 1:51–59
Hodge S, Powell G (2008) Do plant viruses facilitate their aphid vectors by inducing symptoms that alter behavior and performance? Environ Entomol 37:1573–1581
Hodge S, Powell G (2010) Conditional facilitation of an aphid vector, Acyrthosiphon pisum, by the plant pathogen, Pea enation mosaic virus. J Insect Sci 10:155
Inbar M, Doostdar H, Gerling D, Mayer RT (2001) Induction of systemic acquired resistance in cotton by BTH has a negligible effect on phytophagous insects. Entomol Exp Appl 99:65–70
Jensen DD (1959) A plant virus lethal to its insect vector. Virology 8:164–175
Jiu M, Zhou XP, Tong L, Xu J, Yang X, Wan FH, Liu SS (2007) Vector-virus mutualism accelerates population increase of an invasive whitefly. PLoS ONE 1:e182
Kennedy JS (1951) Benefits to aphids from feeding on galled and virus-infected leaves. Nature 168:825
Kluth S, Kruess A, Tscharntke T (2002) Insects as vectors of plant pathogens: mutualistic and antagonistic interactions. Oecologia 133:193–199
Kogovšek P, Pompe-Novak M, Baebler Š, Rotter A, Gow L, Gruden K, Foster GD, Boonham N, Ravnikar M (2010) Aggressive and mild Potato virus Y isolates trigger different specific responses in susceptible potato plants. Plant Pathol 59:1121–1132
Kovač M, Müller A, Milovanovič Jarh D, Milavec M, Düchting P, Ravnikar M (2009) Multiple hormone analysis indicates involvement of jasmonate signalling in the early defence of potato to Potato virus Y NTN. Biol Plant 53:195–199
Krečič-Stres H, Vucak C, Ravnikar M, Kovač M (2005) Systemic Potato virus Y NTN infection and levels of salicylic and gentisic acids in different potato genotypes. Plant Pathol 54:441–447
Kusajima M, Yasuda M, Kawashima A, Nojiri H, Yamane H, Nakajima M, Akutsu K, Nakashita H (2010) Suppressive effect of abscisic acid on systemic acquired resistance in tobacco plants. J Gen Plant Pathol 76:161–167
Leon-Reyes A, Van der Does D, De Lange ES, Delker C, Wasternack C, Van Wees SCM, Ritsema T, Pieterse CMJ (2010) Salicylate-mediated suppression of jasmonate-responsive gene expression in Arabidopsis is targeted downstream of the jasmonate biosynthesis pathway. Planta 232:1423–1432
Mauch-Mani B, Mauch F (2005) The role of abscisic acid in plant-pathogen interactions. Curr Opin Plant Biol 8:409–414
Mauck KE, De Moraes CM, Mescher MC (2010a) Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. Proc Natl Acad Sci USA 107:3600–3605
Mauck KE, De Moraes CM, Mescher MC (2010b) Effects of Cucumber mosaic virus infection on vector and non-vector herbivores of squash. Commun Integr Biol 3:579–582
Mauck KE, Bosque-Pérez NA, Eigenbrode SD, De Moraes CM, Mescher MC (2012) Transmission mechanisms shape pathogen effects on host–vector interactions: evidence from plant viruses. Funct Ecol 26:1162–1175
Mayer RT et al (2002) Multi-trophic interactions of the silverleaf whitefly, host plants, competing herbivores, and phytopathogens. Arch Insect Biochem Physiol 51:151–169
Mehle N, Kovač M, Petrovič N, Novak MP, Baebler Š, Stres HK, Gruden K, Ravnikar M (2004) Spread of potato virus YNTN in potato cultivars (Solanum tuberosum L.) with different levels of sensitivity. Physiol Mol Plant Pathol 64:293–300
Mello AFS, Olarte RA, Gray SM, Perry KL (2011) Transmission efficiency of Potato virus Y strains PVYO and PVYN–Wi by five aphid species. Plant Dis 95:1279–1283
Mowry TM, Ophus JD (2006) Influence of the Potato leafroll virus and virus infected plants on the arrestment of the aphid, Myzus persicae. J Insect Sci 6:22
Muller CB, Williams IS, Hardie J (2001) The role of nutrition, crowding and interspecific interactions in the development of winged aphids. Ecol Entomol 26:330–340
Nanayakkara UN, Nie X, Giguère M, Zhang J, Boquel S, Pelletier Y (2012) Aphid feeding behavior in relation to Potato virus Y (PVY) acquisition. J Econ Entomol 105:1903–1908
Nault LR (1997) Arthropod transmission of plant viruses: a new synthesis. Ann Entomol Soc Am 90:521–541
Ng JCK, Falk BW (2006) Virus–vector interactions mediating nonpersistent and semi-persistent transmission of plant viruses. Annu Rev Phytopathol 44:193–212
Ng JCK, Perry KL (2004) Transmission of plant viruses by aphid vectors. Mol Plant Pathol 5:505–511
Pan X, Welti R, Wang X (2008) Simultaneous quantification of major phytohormones and related compounds in crude plant extracts by liquid chromatography–electrospray tandem mass spectrometry. Phytochemistry 69:1773–1781
Preston CA, Lewandowski C, Enyedi AJ, Baldwin IT (1999) Tobacco mosaic virus inoculation inhibits wound-induced jasmonic acid-mediated responses within but not between plants. Planta 209:87–95
Raubenheimer D, Simpson SJ (1992) Analysis of covariance––an alternative to nutritional indexes. Entomol Exp Appl 62:221–231
Ryals J, Uknes S, Ward E (1994) Systemic acquired-resistance. Plant Physiol 104:1109–1112
Sano H, Ohashi Y (1995) Involvement of small gtp-binding proteins in defense signal-transduction pathways of higher-plants. Proc Natl Acad Sci USA 92:4138–4144
Scholthof KG et al (2011) Top ten plant viruses in molecular plant pathology. Mol Plant Pathol 12:938–954
Shalitin D, Wolf S (2000) Cucumber mosaic virus infection affects sugar transport in melon plants. Plant Physiol 123:597–604
Singh RP et al (2008) Discussion paper: the naming of Potato virus Y strains infecting potato. Arch Virol 153:1–13
Srinivasan R, Alvarez JM (2007) Effect of mixed viral infections (Potato virus Y-Potato leafroll virus) on biology and preference of vectors Myzus persicae and Macrosiphum euphorbiae (Hemiptera: Aphididae). J Econ Entomol 100:646–655
Stout MJ, Thaler JS, Thomma B (2006) Plant-mediated interactions between pathogenic microorganisms and herbivorous arthropods. Annu Rev Entomol 51:663–689
Sylvester ES (1980) Circulative and propagative virus transmission by aphids. Annu Rev Entomol 25:257–286
Thaler JS, Bostock RM (2004) Interactions between abscisic-acid-mediated responses and plant resistance to pathogens and insects. Ecology 85:48–58
Thaler JS, Fidantsef AL, Duffey SS, Bostock RM (1999) Trade-offs in plant defense against pathogens and herbivores: a field demonstration of chemical elicitors of induced resistance. J Chem Ecol 25:1597–1609
Thaler JS, Fidantsef AL, Bostock RM (2002) Antagonism between jasmonate- and salicylate-mediated induced plant resistance: effects of concentration and timing of elicitation on defense-related proteins, herbivore, and pathogen performance in tomato. J Chem Ecol 28:1131–1159
Thaler JS, Owen B, Higgins VJ (2004) The role of the jasmonate response in plant susceptibility to diverse pathogens with a range of lifestyles. Plant Physiol 135:530–538
Thaler JS, Agrawal AA, Halitschke R (2010) Salicylate-mediated interactions between pathogens and herbivores. Ecology 91:1075–1082
Thaler JS, McArt SH, Kaplan I (2012) Compensatory mechanisms for ameliorating the fundamental trade-off between predator avoidance and foraging. Proc Natl Acad Sci USA 109:12075–12080
Ton J, Flors V, Mauch-Mani B (2009) The multifaceted role of ABA in disease resistance. Trends Plant Sci 14:310–317
Underwood N, Halpern S, Klein C (2011) Effect of host-plant genotype and neighboring plants on strawberry aphid movement in the greenhouse and field. Am Midl Nat 165:38–49
Verbeek M, Piron PGM, Dullemans AM, Cuperus C, van der Vlugt RAA (2009) Determination of aphid transmission efficiencies for N, NTN and Wilga strains of Potato virus Y. Ann Appl Biol 156:39–49
Walling LL (2000) The myriad plant responses to herbivores. J Plant Growth Regul 19:195–216
Yang JY, Iwasaki M, Machida C, Machida Y, Zhou XP, Chua NH (2008) Beta C1, the pathogenicity factor of TYLCCNV, interacts with AS1 to alter leaf development and suppress selective jasmonic acid responses. Genes Dev 22:2564–2577
Zarate SI, Kempema LA, Walling LL (2007) Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol 143:866–875
Zhang P, Zheng SJ, van Loon JJA, Boland W, David A, Mumm R, Dicke M (2009) Whiteflies interfere with indirect plant defense against spider mites in Lima bean. Proc Natl Acad Sci USA 106:21202–21207
Acknowledgments
We are greatly indebted to Dawn Smith and Stewart Gray’s laboratory for kindly helping us with viral infection and proving the virus strains used in the experiments. We thank Rayko Halitschke for his valuable assistance in the phytohormone analyses, Ordom Huot for his help with laboratory work, and Cesar Rodriguez-Saona and Isgouhi Kaloshian for providing the aphids. This study strongly benefited from comments and suggestions by Anurag Agrawal, Alison Power, Gui Becker, Suzi Claflin, Scott McArt, Ian Kaplan, the editor John Lill, and two anonymous reviewers. This project received financial support from Sigma Xi (Cornell Chapter), the Department of Ecology and Evolutionary Biology, and the Department of Entomology at Cornell. M. F. K. B. is recipient of the Olin fellowship and the CAPES/Fulbright fellowship (BEX 2234-08-4). The experiments comply with the current laws of the USA, in which the experiments were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by John Lill.
Rights and permissions
About this article
Cite this article
Kersch-Becker, M.F., Thaler, J.S. Virus strains differentially induce plant susceptibility to aphid vectors and chewing herbivores. Oecologia 174, 883–892 (2014). https://doi.org/10.1007/s00442-013-2812-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-013-2812-7