Abstract
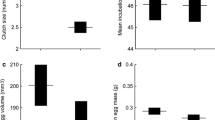
Life history traits may vary within and among species. Rarely, however, are both variations examined concurrently to identify the life history adaptation. We found that female body size, offspring number and size, and incubation period showed convergent evolution in two lacertid lizards (Takydromus wolteri and Eremias argus) that occur sympatrically in high-latitude and low-latitude localities. Females from the high-latitude population were larger and produced larger clutches than those from the low-latitude population. In both species, the incubation period was shorter for the high-latitude population than for the low-latitude population. However, the physiological mechanism underlying the shorter incubation period differed between the species. These results suggest that: (1) sympatric lizards may adopt similar reproductive strategies in response to their common environments, and (2) embryonic development of the two species follows different pathways for adaptation to low temperatures. This study highlights the importance of understanding the adaptive evolution of life history in response to environmental changes at the embryonic life stages.



Similar content being viewed by others
References
Andrews R (2004) Patterns of embryonic development. In: Deeming DC (ed) Reptilian incubation: environment, evolution and behaviour. Nottingham University Press, Nottingham, pp 75–102
Angilletta MJ (2009) Thermal adaptation: a theoretical and empirical synthesis. Oxford University Press, Oxford
Angilletta MJ, Niewiarowski PH, Dunham AE, Leache AD, Porter WP (2004) Bergmann’s clines in ectotherms: illustrating a life-history perspective with sceloporine lizards. Am Nat 164:E168–E183
Angilletta MJ, Oufiero CE, Leache AD (2006) Direct and indirect effects of environmental temperature on the evolution of reproductive strategies: an information-theoretic approach. Am Nat 168:E123–E135
Ashton KG, Feldman CR (2003) Bergmann’s rule in nonavian reptiles: turtles follow it, lizards and snakes reverse it. Evolution 57:1151–1163
Blackledge TA, Gillespie RG (2004) Convergent evolution of behavior in an adaptive radiation of Hawaiian web-building spiders. PNAS 101:16228–16233
Denno RF, Dingle H (1981) Insect life history patterns: habitat and geographic variation. Springer, New York
Du WG, Radder RS, Sun B, Shine R (2009) Determinants of incubation period: do reptilian embryos hatch after a fixed total number of heart beats? J Exp Biol 212:1302–1306
Du WG, Warner DA, Langkilde T, Robbins T, Shine R (2010a) The physiological basis of geographic variation in rates of embryonic development within a widespread lizard species. Am Nat 176:522–528
Du WG, Ye H, Zhao B, Warner DA, Shine R (2010b) Thermal acclimation of heart rates in reptilian embryos. PLoS ONE 5(12):e15308
Du WG, Ye H, Zhao B, Pizzatto L, Ji X, Shine R (2011) Patterns of interspecific variation in the heart rates of embryonic reptiles. PLoS ONE 6:e29027
Du WG, Warner DA, Langkilde T, Robbins T, Shine R (2012) The roles of pre- and post-hatching growth rates in generating a latitudinal cline of body size in the eastern fence lizard (Sceloporus undulatus). Biol J Linn Soci 106:202–209
Dufaure JP, Hubert J (1961) Table de développement du lézard vivipare: Lacerta (Zootoca) vivipara Jacquin. Arch Anat Microsc Morphol Exp 50:309–328
Ewert MA (1985) Embryology of turtles. In: Gans C, Billett F, Maderson PFA (eds) Biology of the reptilia, vol 14. Wiley, New York, pp 75–268
Fischer K, Brakefield PM, Zwaan BJ (2003) Plasticity in butterfly egg size: why larger offspring at lower temperatures? Ecology 84:3138–3147
Fu JZ (2000) Toward the phylogeny of the family Lacertidae—why 4708 base pairs of mtDNA sequences cannot draw the picture. Biol J Linn Soci 71:203–217
Gleiss AC et al (2011) Convergent evolution in locomotory patterns of flying and swimming animals. Nat Commun 2. doi:10.1038/ncomms1350
Hao QL, Liu HX, Ji X (2006) Phenotypic variation in hatchling Mongrolian racerunners Eremias argus from eggs incubated at constant versus fluctuating temperatures. Acta Zool Sin 52:1049–1057
Ji X, Du WG, Qu YF, Lin LH (2009) Nonlinear continuum of egg size-number trade-offs in a snake: is egg-size variation fitness related? Oecologia 159:689–696
Liefting M, Hoffmann AA, Ellers J (2009) Plasticity versus environmental canalization: population differences in thermal responses along a latitudinal gradient in Drosophila serrata. Evolution 63:1954–1963
Losos JB (2000) Ecological character displacement and the study of adaptation. PNAS 97:5693–5695
Losos JB (2011) Convergence, adaptation, and constraint. Evolution 65:1827–1840
Losos JB, Jackman TR, Larson A, de Queiroz K, Rodriguez-Schettino L (1998) Contingency and determinism in replicated adaptive radiations of island lizards. Science 279:2115–2118
Matos M, Simoes P, Duarte A, Rego C, Avelar T, Rose MR (2004) Convergence to a novel environment: comparative method versus experimental evolution. Evolution 58:1503–1510
Niewiarowski PH (1994) Understanding geographic life-history variation in lizards. In: Vitt LJ, Pianka ER (eds) Lizard ecology: historical and experimental perspectives. Princeton University Press, Princeton, pp 31–50
Niewiarowski PH, Angilletta MJ (2008) Countergradient variation in embryonic growth and development: do embryonic and juvenile performances trade off? Funct Ecol 22:895–901
Ohlberger J, Mehner T, Staaks G, Hölker F (2008) Temperature-related physiological adaptations promote ecological divergence in a sympatric species pair of temperate freshwater fish, Coregonus spp. Funct Ecol 22:501–508
Oro D, Pradel R, Lebreton J-D (1999) Food availability and nest predation influence life history traits in Audouin’s gull, Larus audouinii. Oecologia 118:438–445
Oufiero CE, Angilletta MJ (2006) Convergent evolution of embryonic growth and development in the eastern fence lizard (Sceloporus undulatus). Evolution 60:1066–1075
Pan Z-C, Ji X (2001) The influence of incubation temperature on size, morphology and locomotor performance of hatchling grass lizards (Takydromus wolteri). Acta Ecol Sin 21:2031–2038
Parker GA, Begon M (1986) Optimal egg size and clutch size: effects of environment and maternal phenotype. Am Nat 128:573–592
Sears MW, Angilletta MJ (2004) Body size clines in Sceloporus lizards: proximate mechanisms and demographic constraints. Int Comp Biol 44:433–442
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Warner DA, Shine R (2007) Fitness of juvenile lizards depends on seasonal timing of hatching, not offspring body size. Oecologia 154:65–73
Yampolsky LY, Scheiner SM (1996) Why larger offspring at lower temperatures? A demographic approach. Am Nat 147:86–100
Zanette L, Clinchy M, Smith JNM (2006) Food and predators affect egg production in song sparrows. Ecology 87:2459–2467
Zhao EM, Zhao KT, Zhou KY (1999) Fauna sinica reptilia, vol 2 Squamata. Chinese Science Press, Beijing
Acknowledgments
We thank P. Liu, Y. Wang, Z. Y. Zhang and W. B. Yan for their assistance in the field or laboratory. We are grateful to T. R. Robinns, J.-F. Le Galliard and one anonymous reviewer for their comments on the manuscript. This work was supported by grants from the Natural Science Foundation of China (project no. 30770274), the One Hundred Talents Program of the Chinese Academy of Sciences to W.-G. Du and the local government of Anhui Province to X.-F. Xu (KJ2010A249).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jean-François Le Galliard.
Rights and permissions
About this article
Cite this article
Sun, BJ., Li, SR., Xu, XF. et al. Different mechanisms lead to convergence of reproductive strategies in two lacertid lizards (Takydromus wolteri and Eremias argus). Oecologia 172, 645–652 (2013). https://doi.org/10.1007/s00442-012-2524-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-012-2524-4