Abstract
Flower characteristics have been traditionally considered relatively constant within species. However, there are an increasing number of examples of variation in flower characteristics. In this study, we examined the variation in attracting and rewarding flower characters at several ecological levels in a metapopulation of Pyrus bourgaeana in the Doñana area (SW Spain). We answered the following questions: what are the variances of morphological and nectar characters of flowers? How important are intra-individual and inter-individual variance in flower characters? Are there microgeographical differences in flower characters? And if so, are they consistent between years? In 2008 and 2009, we sampled flowers of 72 trees from five localities. For six flower morphological and two nectar characteristics, we calculated coefficients of variation (CV). The partitioning of total variation among-localities, among-individuals, and within-individuals was estimated. To analyze differences among localities and their consistency between years, we conducted generalized linear mixed models. The CVs of nectar characters were always higher than those of morphological characters. As expected, inter-individual variation was the main source of variation of flower morphology, but nectar characters had significant variation at both intra- and inter-individual levels. For most floral traits, there were no differences among localities. Our study documents that variation is a scale-dependent phenomenon and that it is essential to consider intra- and inter-individual variance when investigating the causes and consequences of variation. It also shows that single year studies of floral characters should be viewed with caution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The phenotype of a plant species can change over time and can differ among individuals in different locations (Linhart and Grant 1996; Gómez et al. 2009; Hodgins and Barrett 2008; Herrera 2009). Flower characteristics have been traditionally considered relatively constant because variation may constrain reproductive function (Berg 1960; Brock and Weinig 2007). However, there are numerous examples of variation in flower characteristics among localities (Cresswell 1998; Linhart and Grant 1996; Schlumpberger et al. 2009; Baghalian et al. 2010), among individuals within a locality (Herrera 1990; Boose 1997), and within individuals (Williams and Conner 2001; Garrido et al. 2005; Bateman and Rudall, 2006; Herrera 2009). There is also evidence of change in flower characteristics over the course of the flowering season (Ashman 1992; Worley et al. 2000; Williams and Conner 2001; Herrera 2009).
Ecological and evolutionary factors may cause plant character variability (Williams and Conner 2001; Garrido et al. 2005; Messier et al. 2010). Among localities, variation may be due to local adaptation and/or phenotypic plasticity in response to local conditions. Among individuals, variation may be due to genetic variability and/or phenotypic plasticity in response to fine-scale environmental conditions. Within individuals, variation may be due to organ-level responses to micro-environmental factors (e.g., light availability), differences in tissue age, spatial variation in nutrient availability combined with sectorial transport, and/or mutations (Orians et al. 2002; Obeso 2004; Herrera 2009).
Knowledge of flower traits variation at multiple hierarchical levels is therefore needed to identify the causes of this variation and to assess the potential for selection by animal mutualists (e.g., pollinators and dispersers; Herrera et al. 2002; Ashman and Majetic 2006; Hodgins and Barrett 2008). For example, greater phenotypic variation within than among individuals could weaken selection pressure and evolutionary outcomes (Williams and Conner 2001). On the other hand, the magnitude of variation may also be under selection, because animal counterparts usually select against intra-individual variance (Herrera 2009).
Many flower characters of insect-pollinated species have attracting and rewarding functions. Attracting characters are, e.g., shape, size, color, and scent. Flowers reward visitors with nectar and/or pollen (Pellmyr 2006), the most important factors for pollinator preference. However, flowering plants must balance the costs of nectar production with the benefits of cross-pollination (Schoonhoven et al. 2005; Pellmyr 2006). Attracting and rewarding characters are likely to show different patterns of variation, and the magnitude of their variance might have different consequences for plant fitness (Herrera 2009).
In the present study, we examined the variation in attracting and rewarding flower characters at several ecological levels in a metapopulation of Pyrus bourgaeana (Rosaceae) from a Mediterranean scrubland of southwestern Spain. As attracting characters, we studied flower morphology, and as reward characters, we studied nectar quantity and sugar concentration. Pollinators are expected to avoid individuals with large variance in flower characters (Herrera 2009); hence, we hypothesize that intra-individual variance of floral morphological characters will be relatively small. Pollinators only interact with nectar characters when already attracted by a plant, so we hypothesize that intra-individual variance of nectar characters will be higher than that of morphological characters. Additionally, nectar characters are highly plastic in response to biotic and abiotic factors and change over time (Boose 1997; Mitchell 2004), so we hypothesize that there will be high inter-individual variance in nectar characters.
Partial isolation of populations can give rise to local adaptation (Kawecki and Ebert 2004). However, in our study area, population reduction and fragmentation of P. bourgaeana began only ~200 years ago. Thus, we expect small differences in floral characters among our five localities. However, some of the overall variability of flowers may be due to environmental variations that act on phenotypic plasticity. In Mediterranean regions, water availability is one of the most important factors for plant growth and survival (Thompson 2005a), and can influence floral characters (Lambrecht and Dawson 2007). In our study sites, there are substantial differences in annual rainfall (Fedriani et al. 2010). Thus, we hypothesize that there will be large annual differences in P. bourgaeana floral characters. Simultaneously, our study regions differ in groundwater level (Instituto Tecnologico Geominero Español 1992), so trees from different localities may differ in sensitivity to water deficit. Therefore, we hypothesize that inter-annual differences in inflorescence characters will not be consistent among localities.
The main aim of this study was to assess the different levels of variation in floral morphology and nectar characters in a metapopulation of P. bourgaeana. Specifically, we sought to answer the following questions: what are the variances of morphological and nectar characters of flowers? How important are intra-individual and inter-individual variance in flower characters? Are there microgeographical differences in flower characters? And if so, are they consistent between years?
Materials and methods
Study plant and site
Pyrus bourgaeana is a deciduous monoecious tree, typically 3–6 m tall, which is indigenous to the Iberian Peninsula (Spain, Portugal) and North Africa (Morocco) (Aldasoro et al. 1996). It flowers during February–March, is self-incompatible (authors, unpublished data), and is pollinated by numerous Hymenoptera, Diptera, and Coleoptera species (Herrera 1988; authors, unpublished data). Flowers have radial symmetry (actinomorphic) and five oval petals. A morphometric study of the genus Pyrus in southwest Europe and north Africa indicated that P. bourgaeana has a petal length of 5.4–12.0 mm (mean 8.7 mm), sepal length of 3.4–7.2 mm (mean 5.3 mm), and that flowers have 20–25 stamens and five styles (Aldasoro et al. 1996). However, data on variation at the local, individual, and intra-individual levels are lacking. Each tree produces between 200 and 450 fleshy fruits that ripen from September to December, and their seeds are dispersed by semifrugivorous mammals (Fedriani et al. 2010).
P. bourgaeana is a controversial species from a taxonomic point of view. Browicz (1993) suggested that it is one of the extreme forms of P. communis s.l. Although clear separation of P. bourgaeana and P. communis is difficult, it is possible. P. communis and P. bourgaeana occur in different geographic areas, and have different petal lengths (Aldasoro et al. 1996). Thus, studying intraspecific variation of P. bourgaeana floral traits could contribute to solve this controversy.
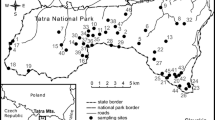
Our focal metapopulation is located in the Doñana area (37°9′N, 6°26′W; elevation 0–80 m), on the west bank of the Guadalquivir river estuary in southwestern Spain. Trees occur at low densities (generally less than one individual per ha; Fedriani et al. 2010) in patches of Mediterranean scrubland that are isolated from each other by natural or anthropogenic barriers (marshes, sand dunes or cultivations). The climate is Mediterranean sub-humid, and is characterized by dry, hot summers (June–September) and mild, wet winters (November–February). Annual rainfall varies widely, and during the last 25 years has ranged from 170 to 1,028 mm (mean ± SD, 540 ± 63 mm). Most rain (80%) occurs from October to March. For the two studied flowering seasons, there was a large difference in precipitation; from October 2007 to March 2008 it was 312 mm, and from October 2008 to March 2009 it was 398 mm.
During the flowering seasons of 2008 and 2009, we sampled flowers of P. bourgaeana trees from five localities in the Doñana area, which are 3–15 km apart (Hato Ratón, Hinojos, Matasgordas, Rocina, and Vera). In Matasgordas and Hato Ratón, there are scattered Quercus suber, Olea europaea var. sylvestris, Fraxinus angustifolia, and Pinus pinea trees, and the understory is dominated by Pistacia lentiscus shrubs growing singly or in small clumps separated by unvegetated sandy substrate or sparse Halimium halimifolium, Ulex spp., and Chamaerops humilis. Rocina is situated in the vicinity of the Rocina stream, and its vegetation is similar to Matasgordas and Hato Ratón, but its understory is dominated by Halimium halimifolium. In Vera, there are scattered Quercus suber and Pinus pinea trees, and an understory of Halimium halimifolium, Ulex spp. and Erica sp. Hinojos is a Pinus pinea forest with open scrublands of Chamaerops humilis and Halimium halimifolium (Valverde 1958; Fedriani et al. 1998, 2010).
Flower morphology
During each sampling season, we collected flowers from 8 to 15 trees per locality (Online Resource 1), in most cases from the same individuals in both seasons. From each tree, five inflorescences (when available) were collected and immediately delivered to the laboratory. Two flowers were randomly selected from each inflorescence for measurements (Online Resource 1).
We took photos of all flowers within a few hours of collection. For each flower we estimated petal length, petal area, corolla area, and calyx area using ImageJ software (e.g., Brock and Weinig 2007). Petal measurements were taken from two randomly selected non-sequential petals per flower. Corolla area was estimated by multiplying the number of petals by the mean petal area (mean area of two measured petals). Calyx area was defined as the area of the calyx maximal aperture. The number of flowers per inflorescence and the number of petals per flower were also recorded.
Quantity of nectar and concentration of nectar sugar
Nectar characteristics were determined, but not from the same flowers as used for measuring morphology, because the large number of sampled flowers and their fragility made it impossible to perform both measurements in sufficiently short time. At each locality, we sampled 4–11 trees, the same trees as used for morphological measurements (Online Resource 1). To eliminate the effect of nectar consumers, in early spring, three inflorescences of each tree were enclosed with a 1-mm mesh brown tulle. When flowers opened, inflorescences were cut early in the morning and immediately taken to the laboratory.
Nectar quantity and concentration of sugars were estimated within a few hours after collection. From each open flower, nectar was extracted with calibrated microcapillaries (1 and 5 μL; accuracy 0.1 μL) (e.g., Farkas and Orosz-Kovács 2003). If there was at least 1 μL of nectar, the concentration of sugars was measured with a pocket refractometer (range 0–35%; concentration of sugar exceeded 35% in only 3.4% of cases).
Statistical analysis
We calculated coefficients of variation (CV) for morphological and nectar characteristics at the level of the individual, inflorescence and flower. This statistic is particularly useful for comparing the variation of measurements involving different units or samples with different means (Lande 1977). At the individual level, we first estimated the mean per inflorescence and then we estimated the grand mean (i.e. the mean of the mean value per individual). And similarly, at the inflorescence level, we first estimated the mean of two petals per flower and than we estimated the grand mean per inflorescence.
To estimate the partitioning of total variation of particular floral characteristics among-localities, among-individuals, and within-individuals (among inflorescences and among-flowers) components, we analyzed the variance components. The following levels of variation were considered: locality, tree nested within locality, inflorescence nested within tree and locality, and flower nested within inflorescence, tree, and locality. All levels were considered as random effects, as required for variance partitioning. Analyses were conducted with the mixed procedure of SAS (2005, SAS Institute).
To analyze differences among localities and their consistency between years, we conducted generalized linear mixed models using the macro GLIMMIX (Littell et al. 2006) in SAS (2005, SAS Institute). Only five localities and 2 years were used for this study, so it cannot be assumed that our sample was an adequate random sample of all locations within Doñana and of all years. Therefore, models were fitted with locality and year and their interactions as fixed effects (Rey et al. 2006). Tree nested within locality, inflorescence nested within tree, and flower nested within inflorescence were included as random factors. The variable concentration of sugar in nectar was arcsin-transformed to achieve homogeneity of variances. For number of petals, we used Poisson error, and for the other variables we assumed normal error. Adjusted means and standard errors were calculated using the LSMEANS statement in SAS. To compare the effects of different levels of any significant main factor, we calculated the difference between their least-square means. When the interaction between year and locality was significant, we performed tests for the effect of a given factor at a different level of the other factor (test of simple main effects) using the SLICE option (Littell et al. 2006).
Results
There were a mean of 9.5 ± 0.5 (SE) flowers per inflorescence, with a range of 3–20, and a mean of 5.2 ± 0.15 petals per flower, with a range of range 3–14. Mean petal length was 12.0 ± 0.2 mm (range 6.7–19.6 mm), mean petal area was 80.0 ± 2.8 mm2 (range 23.5–218.0 mm2), mean corolla area was 417.2 ± 35.0 mm2 (range 166.2–1,109.3 mm2), and mean calyx area was 30.7 ± 1.9 mm2 (range 9.9–57.9 mm2). The overall mean amount of nectar per flower was 2.0 ± 0.5 μL (range 0–18.5 μL), and the average nectar concentration was 18.8 ± 0.1% (range 3.0–35.0; Online Resource 2).
Variation within and among individuals
As predicted, the CV of nectar characters were always higher than those of morphological characters (Table 1). The mean CV of all morphological characters was more than twice as high among individuals than within individuals (Fig. 1). The mean CV of the two nectar characters was slightly higher within individuals than among individuals (Fig. 1).
Our data for all morphological characters in both years indicated that most of the variance was explained by differences among individuals (53–79% of total variance; Fig. 2). For instance, for the calyx area, variance among trees explained ~75% of the total variance during both years. Within-individual variation accounted only for a small fraction of the total variance. For the number of petals, corolla area, and calyx area, the within-individual variance explained only 4–12% of the total variance (Fig. 2). For petal characters (longitude and area), the within-individual variance was slightly larger (10–19%). The unexplained part of the variance was high (~40%) for the number of flowers per inflorescence and the number of petals per flower; in contrast, for petal size characters, the unexplained variance was only ~10%.
Variance components (among localities, trees, inflorescences and flowers) for floral morphological and nectar characters of Pyrus bourgaeana. Statistically significant variation (P < 0.05) among localities, trees, inflorescences and flowers is indicated by an asterisk within the corresponding bar of a column
Our data for nectar characters indicated that the level of explained variance depended on the specific character, and that there were differences in the 2 years. Generally, variation among individuals explained less of the total variance (range 0–35%) than for morphological characters; and in contrast, variation within-individuals explained more of the total variance than for morphological characters. For both characters (nectar quantity and sugar concentration), the within-individual variance was the major cause of variability, and even accounted for 43% of the total variance in 1 year. A considerable part of the variance (higher than for morphological characters) was unexplained for both nectar characters (Fig. 2).
Differences among localities and their consistency between years
There were no differences among localities in the number of flowers per inflorescence when we accounted for random effects. However, this character varied significantly between the 2 years, and there were slightly more flowers per inflorescence in 2009 than 2008 (Table 2; Online Resource 2). The lack of significant interactions between years and localities indicated that inter-annual differences were consistent among localities.
There were no differences in the number of petals per flower among localities or between years. The longest and shortest petals were in Matasgordas and Vera, respectively (Online Resource 2). For petal length, locality had a significant effect (Table 2), but there was a significant interaction between year and locality, indicating that differences among localities were not consistent between the years. Tests of slices indicated that differences among localities were significant during 2008 (F 4,725 = 5.0, P < 0.001) but not 2009 (P = 0.277). For example, during 2008, Hato Ratón had a longer mean petal length (12.4 mm) than Rocina (11.1 mm); however, during 2009, there was no difference (Online Resource 2). The area of petals generally did not differ among localities (Table 2), and we observed differences among localities only in 2008 (F 4,725 = 2.94, P = 0.020). The petal area was slightly but significantly higher during 2008 than 2009 (Table 2).
Overall, corolla area did not differ among localities (Table 2), but the interaction between locality and year was significant. In 2008, but not 2009, there were marginally significant differences among localities (F 4,725 = 2.34, P = 0.054), with Matasgordas and Hinojos having the largest and smallest flowers, respectively (Online Resource 2).
Calyx area was noticeably larger in Vera than in other localities, although locality and year had no significant effects as main factors (Table 2). The interaction between locality and year was significant and there were differences among localities in 2009 (F 4,725 = 2.84, P = 0.023), but not 2008 (P = 0.342).
We found significant differences among localities in the quantity of nectar (Table 2). The highest and lowest quantities of nectar were in Hato Ratón and Matasgordas, respectively. Year did not have an effect as a main factor, but there was a significant interaction between year and locality, indicating that differences among localities were not consistent between years. For example, during 2008, the mean quantity of nectar was similar in Hato Ratón and Rocina; however, during 2009, the mean quantity of nectar in Hato Ratón was almost double that of Rocina (Online Resource 2).
There were only marginal differences in sugar concentration in nectar among localities (P = 0.057; Table 2), but there was a significant interaction between year and locality (Table 2). Tests of slices indicated significant differences among localities in both years (2008: F 4,420 = 5.94, P = 0.0001; 2009: F 4,420 = 7.0, P < 0.0001), but the direction of differences was not consistent between the 2 years. For example, in 2008, the highest nectar concentration was in Vera, but in 2009, the highest nectar concentration was in Hinojos. Overall, sugar concentration was 1.2-fold higher in 2008 than 2009 and differences between years were significant (Table 2).
Discussion
Our results reveal quantitative variations in the morphology and nectar characteristics of P. bourgaeana flowers at the intra-individual, inter-individual, and microgeographical levels. Thus, although flower characters are traditionally considered to have low variance, our results support recent reviews (Cresswell 1998; Herrera 2009), which indicate significant intra-specific variance in flower characters.
Variation within and among individuals
In general, our results indicate greater variation of nectar characters than morphological characters, both among and within individuals. In particular, the inter-individual CVs of nectar characters (43%) doubled those for morphological characters (21%). This clearly agrees with a trend reported by Cresswell (1998) across a wide range of taxa; in that study, the mean variation of nectar characters (54%) was about twofold greater than that of corolla morphological characters (22%). Cresswell (1998) concluded that the lower variability of morphological characters was due to selective pressure to preserve the “mechanical fit” of flowers and pollinators. However, in case of P. bourgaeana flowers, “mechanical fit” between flowers and their pollinators seems unlikely because they are not specialized.
Our results showed that intra-individual variation of flower morphological characters were only a minor part of total flower variation. This is in contrast to the conclusions of a recent review by Herrera (2009), who suggested that high intra-plant variation is common in continuous and nearly continuous morphological characters of flowers. In particular, Herrera found that for 97 animal-pollinated plants, the percentage of variance for floral characters was 5.8–100%, and was more than 50% in 27% of species. Our results for flowers of P. bourgaeana suggest that intra-individual flower morphological characters have relatively low variance.
The lower variance of morphological characters than nectar characters presumably results from plant–pollinator interactions. Pollinators tend to avoid individuals with high intra-individual variance in flower characters (Real 1981; Real and Rathcke 1988; Shafir et al. 1999; Biernaskie et al. 2002), and a higher CV is associated with a stronger aversion (Shafir 2000). Within-plant choice of flowers means that insects must invest time in assessing the phenotypic diversity of available flowers, thus reducing foraging efficiency (Herrera 2009). Flower morphological characters attract pollinators; hence, we can assume that the lower intra-individual variance would be associated with a greater chance of pollinator visitation.
A similar trend might be naively expected for nectar, because pollinators would be expected to choose plants with more consistent rewards. However, there is an important difference between variance in nectar and variance in flower morphology, because, in order to test nectar character variability, pollinators must actually approach and sample numerous flowers. While feeding on nectar, insects unintentionally pollinate flowers. Reducing the time that a pollinator spends on a given tree will promote transport of pollen to other trees, thereby providing increased outcrossing and reproductive success. Thus, a high variance of nectar characters forces pollinators to move to other plants (Biernaskie et al. 2002). On the other hand, within-tree variability in nectar can reduce the energy that the plant invests in “rewards”, a strategy known as the “blank-bonanza” pattern (Feinsinger 1978). In this model, flowers with a large amount of nectar (“bonanzas”) are dispersed among many flowers with little or no nectar (“blanks”) so that plants expend less energy per flower and obtain increased pollinators movement and pollen dispersal (Feinsinger 1978). Thus, it is possible that the high intra-individual variability of nectar characters that we observed in P. bourgaeana provides an adaptive advantage for individuals.
Our results indicate that differences among trees were the most important source of variation in flower morphological characters. Similar results have been reported previously for other species (Cresswell 1998; Kearns and Inouye 1993; Møler and Eriksson 1994). Inter-individual variation in flower morphological characters could be an effect of phenotypic plasticity, and related to differences in microhabitat conditions. Previous studies have shown that variability in environmental factors (e.g., water, light, temperature, nutrient level) can influence floral characters (Villarreal and Freeman 1990; Lambrecht and Dawson 2007; Delesalle and Mazer 1996; Vogler et al. 1999; Catley et al. 2002). In our study, the most probable differences in environmental conditions within populations seem to relate to water and nutrient levels. Therefore, further studies assessing the effect of resource variation on floral morphology and nectar are clearly needed.
The inter-individual variation of flower morphological characters that we found for P. bourgaeana could also result from genetic variation. It would suggest a potential to respond to selection (Vogler et al. 1999) which may be important for a fragmented metapopulation with low population density, as in our metapopulation in Doñana. Such fragmentation and low population density often have negative effects on population genetic diversity, and can even cause population decline (Jacquemyn et al. 2003; Arroyo-Rodriguez et al. 2007). If the individual variance among trees has a genetic basis, it would suggest that the metapopulation of P. bourgaeana in Doñana is maintaining its genetic diversity despite fragmentation. Importantly, genetic variation and phenotypic plasticity may both contribute to our observed results.
Our results also revealed that, for nectar characters, the magnitudes of intra- and inter-individual variations were comparable and high, a phenomenon that has been documented for many plant species (Feinsinger 1978; Zimmerman and Pyke 1986; Boose 1997). Nectar characters are highly plastic, and light, water, fertilization, temperature, CO2 concentration, and other factors can have a strong influence (Villarreal and Freeman 1990; Boose 1997; Orians et al. 2002). The intra-individual variance may be due to phenotypic plasticity in organ-level responses to spatial variation of microenvironmental conditions. Resource allocation is another important cause of variation (Obeso 2004; Weiner 2004). Previous studies have shown that nectar characters can change significantly over time (Mitchell 2004 review), so the day-to-day variation in nectar volume and overall variance in nectar volume may exceed the variation among individual plants (Real and Rathcke 1988). Daily high variability of nectar secretion has been reported for Pyrus cultivars (Farkas and Orosz-Kovács 2003). In some cases, variation in the quantity of nectar arises from differences in the number of nectaries (Herrera and Soriguer 1983).
Similar magnitudes of intra- and inter-individual variation in nectar characters could suggest that direct selection by pollinators is unlikely in this case, because inter-individual variation could be difficult for pollinators to assess (Boose 1997; Kawecki and Ebert 2004). However, the level of the variation can itself be under selection, because animals that interact with plants can respond to intra-individual variability and act as selective agents (Pleasants 1983; Boose 1997; Herrera 2009).
Differences among localities and their consistency between years
We found no differences among localities for most floral traits. We can suggest at least four reasons for this result. First, in Doñana, fragmentation of the P. bourgaeana population occurred within the past ~200 years. P. bourgaeana can live more than 100 years (authors, unpublished data), so the time since fragmentation has been too short for significant local selection. Second, it is possible that our localities did not differ sufficiently in either insect assemblages or selective pressures exerted by pollinators. Third, even if selection pressures by pollinators differ among localities, ‘trait remixing’ (sensu Thompson 2005b) among localities would be likely via pollen and/or seed dispersal. Indeed, our target population is actually a metapopulation and, by definition, there is gene flow among localities (i.e. subpopulations), which acts against local adaptation (Kawecki and Ebert 2004). And lastly, P. bourgaeana flowers show radial symmetry and are pollinated by unspecialized pollinators (Hymenoptera, Diptera and Coleoptera; Herrera 1988; authors, unpublished data). Local adaptation is considered unlikely to occur in cases of generalist interactions (Kawecki and Ebert 2004). In such systems, there is frequent temporal variation in the identity and abundance of the most important selective agents, causing strong fluctuations in selective regimes (Waser et al. 1996; Gómez and Zamora 2006; but see Gómez et al. 2008).
Phenotypic plasticity is a likely source of some variability observed among localities (Sultan 2000; Givnish 2002). Environmental heterogeneity favors the evolution of adaptive phenotypic plasticity and leads to adaptive phenotypic differentiation without underlying genetic differentiation (e.g., Williams and Conner 2001; Kawecki and Ebert 2004). On the other hand, at the microhabitat scale, phenotypic plasticity can cause trees of one locality growing in a unique microhabitat to differ in phenotypic response. If there was a high heterogeneity of microhabitats in our localities, this may explain why differences among localities are not apparent.
Moisture availability can be a limiting factor for plants in arid and semi-arid environments (e.g., those in Mediterranean regions; Thompson 2005a). For example, Lambrecht and Dawson (2007) reported that increasing moisture availability increases the area of flowers. Thus, part of the interannual differences in P. bourgaeana floral characters that we observed could be related to differences in water availability in 2008 and 2009. In fact, there was lower rainfall in 2008 than 2009, and we found that the number of flowers per inflorescence was greater in 2009 at all localities. There were also significant differences in the area of petals in 2008 and 2009, but this was not consistent among localities. It seems likely that, because our localities differed in the availability of ground water (water table ranges 8–20 m a.s.l.; Instituto Tecnologico Geominero Español 1992), they were not equally sensitive to changes in rainfall water.
Our results also have implications for Pyrus taxonomical discrimination. Thus, while Aldasoro et al. (1996) estimates of petal length for P. bourgaeana and P. communis ranged from 5.4 to 12.0 mm (mean 8.7 mm) and from 12.0 to 15.0 mm (mean 13.2 mm), respectively, in Doñana, we found that petal length for P. bourgaeana was longer than 12 mm in about 50% of cases. Because of such an overlap between both Pyrus species, discrimination based on floral traits (Aldasoro et al. 1996) should be considered with caution. Other traits (e.g., leaf traits) and, especially, genetic profiles should be considered in future assessments.
In conclusion, we found significant variations in flower morphological and nectar characters of P. bourgaeana at microgeographical, inter-individual, intra-individual, and intra-inflorescence levels. The magnitude of flower phenotypic variation was different at different levels. Therefore, we suggest that, when investigating the causes and consequences of variation, it is important to consider its scale-dependent nature. In particular, it is essential to consider intra- and inter-individual variance. For P. bourgaeana, inter-individual variation was the main source of variation of flower morphology, but nectar characters had significant variation at both intra- and inter-individual levels. We found only small differences among the five studied localities, and these were not consistent between years. Although considerable data are available on long-term quantitative variation of flower set (mast seeding studies), there are few long-term data on qualitative variation of flower characters. Even though our study suggests general constancy on floral traits between years, we also found interannual significant differences for a few traits during the relatively short time frame considered (2 years). Greater differences on floral traits would be most likely found on a longer time span. Therefore, short-term studies of floral characters should be viewed with caution.
References
Aldasoro J, Aedo C, Muñoz Garmendia F (1996) The genus Pyrus L (Rosaceae) in south-west Europe and north Africa. Bot J Linn Soc 121:143–158
Arroyo-Rodriguez V, Aguirre A, Benítez-Malvido J, Mandujano S (2007) Impact of rain forest fragmentation on the population size of a structurally important palm species: Astrocaryum mexicanum at Los Tuxtlas, Mexico. Biol Conserv 138:198–206
Ashman TL (1992) Indirect costs of seed production within and between seasons in a gynodioecious species. Oecologia 92:266–272
Ashman TL, Majetic C (2006) Genetic constraints on floral evolution: a review and evaluation of patterns. Heredity 96:343–352
Baghalian K, Shabani Sheshtam M, Jamshidi AH (2010) Genetic variation and heritability of agro-morphological and phytochemical traits in Iranian saffron (Crocus sativus L.) populations. Ind Crop Prod 31:401–406
Bateman R, Rudall P (2006) Evolutionary and morphometric implications of morphological variation among flowers within an inflorescence: a case-study using European orchids. Ann Bot 98:975–993
Berg RL (1960) The ecological significance of correlation pleiades. Evolution 14:171–180
Biernaskie J, Cartar R, Hurly T (2002) Risk-averse inflorescence departure in hummingbirds and bumble bees: could plants benefit from variable nectar volumes? Oikos 98:98–104
Boose L (1997) Sources of variation in floral nectar production rate in Epilobium canum (Onagraceae): implications for natural selection. Oecologia 110:493–500
Brock M, Weinig C (2007) Plasticity and environment-specific covariances: an investigation of floral-vegetative and within flower correlations. Evolution 61:2913–2924
Browicz K (1993) Conspect and chorology of the genus Pyrus L Arboretum. Kórnickie 38:17–33
Catley J, Brooking I, Davies L, Halligan E (2002) Temperature and irradiance effects on Sanderonia aurantiaca flower shape and pedicel length. Sci Hortic 93:157–166
Cresswell J (1998) Stabilizing selection and the structural variability of flowers within species. Ann Bot 81:463–473
Delesalle VA, Mazer SJ (1996) Nutrient levels and salinity affect gender and floral traits in the autogamous Spergularia marina. Int J Plant Sci 157:621–631
Farkas Á, Orosz-Kovács Zs (2003) Nectar secretion dynamics of Hungarian local pear cultivars. Plant Syst Evol 238:57–67
Fedriani JM, Ferreras P, Delibes M (1998) Dietary response of the Eurasian badger, Meles meles, to a decline of its main prey in the Doñana national park. J Zool 245:214–218
Fedriani JM, Wiegand T, Delibes M (2010) Spatial pattern of adult trees and mammal-generated seed rain in the Iberian pear. Ecography 33(3):545–555
Feinsinger P (1978) Ecological interactions between plants and hummingbirds in a successional tropical community. Ecol Monogr 48:269–287
Garrido J, Rey P, Herrera C (2005) Fuentes de variación en el tamaño de la herbácea perenne Helleborus foetidus L (Ranunculaceae). Ann Jard Bot Madrid 62:115–125
Givnish T (2002) Ecological constraints on the evolution of plasticity in plants. Evol Ecol 16:213–242
Gómez J, Zamora R (2006) Ecological factors that promote the evolution of generalization in pollination systems. In: Waser N, Ollerton J (eds) Plant-pollinator interactions from specialization to generalization. University of Chicago Press, Chicago, pp 145–165
Gómez JM, Bosch J, Perfectti F, Fernández JD, Abdelaziz M, Camacho JPM (2008) Spatial variation in selection on corolla shape in a generalist plant is promoted by the preference patterns of its local pollinators. Proc R Soc Lond B 275:2241–2249
Gómez J, Abdelaziz M, Camacho J, Muñoz-Pajares A, Perfectti F (2009) Local adaptation and maladaptation to pollinators in a generalist geographic mosaic. Ecol Lett 12:1–11
Herrera J (1988) Pollination relationships in southern Spanish Mediterranean shrublands. J Ecol 76:274–287
Herrera C (1990) The adaptedness of the floral phenotype in a relict endemic, hawkmoth-pollinated violet. 1. Reproductive correlates of floral variation. Biol J Linn Soc 40:263–274
Herrera C (2009) Multiplicity in unity. Plant subindividual variation and interaction with animals. University of Chicago Press, Chicago
Herrera C, Soriguer R (1983) Intra- and inter-floral heterogeneity of nectar production in Helleborus foetidus L. (Ranunculaceae). Bot J Linn Soc 86:253–260
Herrera C, Cerdá X, García M, Guitián A, Medrano M, Rey P, Sánchez-Lafuente A (2002) Floral integration, phenotypic covariance structure and pollinator variation in bumblebee-pollinated Helleborus foetidus. J Evol Biol 15:108–121
Hodgins K, Barrett S (2008) Geographic variation in floral morphology and style-morph ratios in a sexually polymorphic daffodil. Am J Bot 95:185–195
Instituto Tecnologico Geominero Español (1992) Hidrogeologia del Parque Nacional de Doñana y su entorno. Instituto Tecnologico Geominero Español, Madrid
Jacquemyn H, Van Rossum F, Brys R, Endels P, Hermy M, Triest L, De Blust G (2003) Effects of agricultural land use and fragmentation on genetics, demography and population persistence of the rare Primula vulgaris, and implications for conservation. Belg J Bot 136:5–22
Kawecki T, Ebert D (2004) Conceptual issues in local adaptation. Ecol Lett 7:1225–1241
Kearns C, Inouye D (1993) Techniques for pollination biologists. University Press of Colorado, Niwot
Lande R (1977) On comparing coefficients of variation. Syst Zool 26:214–277
Lambrecht S, Dawson T (2007) Correlated variation of floral and leaf traits along a moisture availability gradient. Oecologia 151:574–583
Linhart Y, Grant M (1996) Evolutionary significance of local genetic differentiation in plants. Annu Rev Ecol Syst 27:237–277
Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O (2006) SAS for mixed models, 2nd edn. SAS Institute, Cary
Messier J, McGill BJ, Lechowicz MJ (2010) How do traits vary across ecological scales? A case for trait-based ecology. Ecol Lett 13:838–848
Mitchell R (2004) Heritability of nectar traits: why do we know so little? Ecology 85:1527–1533
Møler A, Eriksson M (1994) Patterns of fluctuating asymmetry in flowers: implications for sexual selection in plants. J Evol Biol 7:97–113
Obeso J (2004) A hierarchical perspective in allocation to reproduction from whole plant to fruit and seed level. Perspect Plant Ecol Evol Syst 6:217–225
Orians C, Ardón M, Mohammad B (2002) Vascular architecture and patchy nutrient availability generate within-plant heterogeneity in plant traits important to herbivores. Am J Bot 89:270–278
Pellmyr O (2006) Pollination by animals. In: Herrera CM, Pellmyr O (eds) Plant–animal interaction. An evolutionary approach. Blackwell, Oxford, pp 157–184
Pleasants J (1983) Nectar production patterns in Ipomopsis aggregata (Polemoniaceae). Am J Bot 70:1468–1475
Real L (1981) Uncertainty and pollinator-plant interactions: the foraging behavior of bees and wasps on artificial flowers. Ecology 62:20–26
Real LA, Rathcke BJ (1988) Patterns of individual variability in floral resources. Ecology 69:728–735
Rey P, Herrera C, Guitián C, Cerdá X, Sánchez-Lafuente A, Medrano M, Garrido J (2006) The geographic mosaic in predispersal interactions and selection on Helleborus foetidus (Ranunculaceae). J Evol Biol 19:21–34
Schlumpberger B, Cocucci A, Moré M, Sérsic A, Raguso R (2009) Extreme variation in floral characters and its consequences for pollinator attraction among populations of an Andean cactus. Ann Bot 103:1489–1500
Schoonhoven L, van Loon J, Dicke M (2005) Insect–plant biology. Oxford University Press, Oxford
Shafir S, Wiegmann D, Smith B, Real L (1999) Risk-sensitive foraging: choice behaviour of honeybees in response to variability in volume of reward. Anim Behav 57:1055–1061
Shafir S (2000) Risk-sensitive foraging: the effect of relative variability. Oikos 88:663–669
Sultan S (2000) Phenotypic plasticity for plant development, function and life history. Trends Plant Sci 5:537–542
Thompson JN (2005a) Plant evolution in the Mediterranean. Oxford University Press, Oxford
Thompson JN (2005b) The geographic mosaic of coevolution. University of Chicago Press, Illinois
Valverde JA (1958) An ecological sketch of the Coto Doñana. Br Birds 51:1–23
Villarreal A, Freeman E (1990) Effects of temperature and water stress on some floral nectar characteristics in Ipomopsis longiflora (Polemoniaceae) under controlled conditions. Bot Gaz 151:5–9
Vogler D, Peretz A, Stephenson A (1999) Floral plasticity in an iteroparous plant: the interactive effects of genotype, environment, and ontogeny in Campanula rapunculoides (Campanulaceae). Am J Bot 86:482–494
Waser N, Chittka L, Price M, Williams N, Ollerton J (1996) Generalization in pollination systems, and why it matters. Ecology 77:1043–1060
Weiner J (2004) Allocation, plasticity and allometry in plants. Perspect Plant Ecol Evol Syst 6:207–215
Williams J, Conner J (2001) Sources of phenotypic variation in floral traits in wild radish, Raphanus raphanistrum (Brassicaceae). Am J Bot 88:1577–1581
Worley A, Baker A, Thompson J, Barrett S (2000) Floral display in Narcissus: variation in flower size and number at the species, population and individual levels. Int J Plant Sci 161:69–79
Zimmerman M, Pyke G (1986) Reproduction in Polemonium: patterns and implications of floral nectar production and standing crops. Am J Bot 73:1405–1415
Acknowledgments
The authors thank to Gemma Calvo and volunteers for their enthusiastic field and laboratory assistance. The Spanish Ministerio de Medio Ambiente (070/2009 grant), Ministerio de Educación y Ciencia (CGL2007-63488/BOS; CGL2010-21926/BOS) and Ministerio de Ciencia e Innovación (ICTS-2009-39) supported this study. Our study complies with the current laws of Spain as well as the rules of the Doñana National Park, where the sampling was carried out.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jeff Karron.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Żywiec, M., Delibes, M. & Fedriani, J.M. Microgeographical, inter-individual, and intra-individual variation in the flower characters of Iberian pear Pyrus bourgaeana (Rosaceae). Oecologia 169, 713–722 (2012). https://doi.org/10.1007/s00442-011-2232-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-011-2232-5