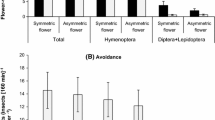
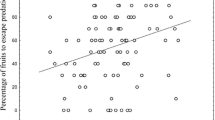
Abstract
Flowers attract insects and so are commonly exploited as foraging sites by sit-and-wait predators. Such predators can be costly to their host plant by consuming pollinators. However, sit-and-wait predators are often prey generalists that also consume plant antagonists such as herbivores, nectar robbers and granivores, so may also provide benefits to their host plant. Here we present a simple, but general, model that provides novel predictions about how costs and benefits interact in different ecological circumstances. The model predicts that the ecological conditions in which flower-dwelling predators are found can generate either net benefits to their host plants, net costs to their host plants, or can have no effect on the fitness of their host plants. The net effect is influenced by the relative densities of mutualists and antagonists. The flower-dwelling predator has a strong positive effect on the plant if both the pollinators and the granivores are at high density. Further, the range of density combinations that yield a positive net outcome for the plant increases if the performance of pollinators is negatively density dependent, if the predator is only moderately effective at influencing flower visitor rates by its potential prey, and if pollinators are very effective. If plants of a given species find themselves consistently in conditions where they benefit from the presence of a predator then we predict that natural selection could favour the evolution of plant traits that increase the likelihood of predator recruitment and retention, especially where plants are served by highly effective pollinators.





Similar content being viewed by others
References
Abbott KR, Dukas R (2009) Honeybees consider flower danger in their waggle dance. Anim Behav 78:633–635
Anderson RA, Karasov WH (1981) Contrasts in energy-intake and expenditure in sit-and-wait and widely foraging lizards. Oecologia 49:67–72
Carter PE, Rypstra AL (1995) Top-down effects in soybean agroecosystems: spider density affects herbivore damage. Oikos 72:433–439
Chase JM, Abrams PA, Grover JP, Diehl S, Chesson P, Holt RD, Richards SA, Nisbet RM, Case TJ (2002) The interaction between predation and competition: a review and synthesis. Ecol Lett 5:302–315
Chittka L (2001) Camouflage of predatory crab spiders on flowers and the colour perception of bees (Aranida: Thomisidae/Hymenoptera: Apidae). Entomol Gen 25:181–187
Chittka L, Thomson JD, Waser NM (1999) Flower constancy, insect psychology, and plant evolution. Naturwissenschaften 86:361–377
Darwin C (1876) On the effects of cross and self fertilisation in the vegetable kingdom. Murray, London
Denno RF, Gratton C, Peterson MA, Langellotto GA, Finke DL, Huberty AF (2002) Bottom-up forces mediate natural-enemy impact in a phytophagous insect community. Ecology 83:443–1458
Dukas R (2001) Effects of perceived danger on flower choice by bees. Ecol Lett 4:327–333
Dukas R (2005) Bumblebee predators reduce pollinator density and plant fitness. Ecology 86:1401–1406
Dukas R, Morse DH (2003) Crab spiders affect flower visitation by bees. Oikos 101:157–163
Dukas R, Morse DH (2005) Crab spiders show mixed effects on flower-visiting bees and no effect on plant fitness components. Ecoscience 12:244–247
Dukas R, Morse DH, Myles S (2005) Experience levels of individuals in natural bee populations and their ecological implications. Can J Zool 83:492–497
Feldman TS (2006) Pollinator aggregative and functional responses to flower density: does pollinator response to patches of plants accelerate at low densities? Oikos 115:128–140
Goncalves-Souza T, Omena PM, Souza JC, Romero GQ (2008) Trait-mediated effects on flowers: artificial spiders deceive pollinators and decrease plant fitness. Ecology 89(9):2407–2413
Goulson D (2003) Bumblebees: behaviour and ecology. Oxford University Press, Oxford
Halaj J, Wise DH (2001) Terrestrial trophic cascades: how much do they trickle? Am Nat 157:262–281
Heiling AM, Herberstein ME, Chittka L (2003) Crab spiders manipulate flower signals. Nature 421:334
Higginson AD (2005) Effects of wing damage on the foraging behaviour of the honeybee Apis mellifera. PhD thesis, University of Nottingham, Nottingham
Hurd LE (1999) Ecology of praying mantids. In: Prete FR, Wells H, Wells PH, Hurd LE (eds) The praying mantids. Johns Hopkins University Press, Baltimore
Ings TC, Chittka L (2008) Speed-accuracy tradeoffs and false alarms in bee responses to cryptic predators. Curr Biol 18:1520–1524
Ings TC, Chittka L (2009) Predator crypsis enhances behaviourally mediated indirect effects on plants by altering bumblebee foraging preferences. Proc R Soc Lond B 276:2031–2036
Knight TM, Chase JM, Hillebrand H, Holt RD (2006) Predation on mutualists can reduce the strength of trophic cascades. Ecol Lett 9:1173–1178
Linton MC, Crowley PH, Williams JT, Dillon PM, Aral H, Strohmeier KL, Wood C (1991) Pit relocation by antlion larvae—a simple-model and laboratory test. Evol Ecol 5:93–104
Louda SM (1982) Inflorescence spiders: a cost/benefit analysis for the plant Haplopappus venetus Blake (Asteraceae). Oecologia 55:185–191
Marquis RJ (1984) Leaf herbivores decrease fitness of a tropical plant. Science 226:537–539
Matsura T, Inoue T (1999) The ecology and foraging strategy of Tenodera augustipennis. In: Prete FR, Wells H, Wells PH, Hurd LE (eds) The praying mantids. Johns Hopkins University Press, Baltimore
Melián CJ, Bascompte J, Jordana P, Křivan V (2009) Diversity in a complex ecological network with two interaction types. Oikos 118:122–130
Memmott J (1999) The structure of a plant-pollinator food web. Ecol Lett 2:276–280
Memmott J, Waser NM, Price MV (2004) Tolerance of pollination networks to species extinctions. Proc R Soc Lond B 10:710–717
Moran MD, Scheidler AR (2002) Effects of nutrients and predators on an old-field food chain: interactions of top-down and bottom-up processes. Oikos 98:116–124
Morse DH (1986) Predatory risk to insects foraging at flowers. Oikos 46:223–228
Morse DH (1993) Choosing hunting sites with little information: patch choice responses of crab spiders to distant cues. Behav Ecol 4:61–65
Morse DH (1999) Choice of hunting site as a consequence of experience in late-instar crab spiders. Oecologia 120:252–257
Morse DH (2007) Predator upon a flower: life history and fitness in a crab spider. Harvard University Press, Cambridge
Morse DH, Fritz (1982) Experimental and observational studies of patch choice at different scales by the crab spider Misumena vatia. Ecology 63:172–182
Mothershead K, Marquis RJ (2000) Fitness impacts of herbivory through indirect effects on plant-pollinator interactions in Oenothera macrocarpa. Ecology 81:30–40
Muñoz AA, Arroyo ATK (2004) Negative impacts of a vertebrate predator on insect pollinator visitation and seed output in Chuquiraga oppositifolia, a high Andean shrub. Oecologia 138:66–73
Olive CW (1982) Behavioral response of a sit-and-wait predator to spatial variation in foraging gain. Ecology 63:912–920
Raine NE, Chittka L (2005) Comparison of flower constancy and foraging performance in three bumblebee species. Entomol Gen 28:81–89
Reader T, Higginson AD, Barnard CJ, Gilbert FS (2006) The effects of predation risk from crab spiders on bee foraging behaviour. Behav Ecol 17:933–939
Riechert SE, Bishop L (1990) Prey control by an assemblage of generalist predators: spiders in garden test systems. Ecology 71:1441–1450
Robertson IC, Maguire DK (2005) Crab spiders deter insect visitations to slickspot peppergrass flowers. Oikos 109:577–582
Romero GQ, Vasconcellos-Neto J (2004) Beneficial effects of flower-dwelling predators on their host plant. Ecology 85:446–457
Ruhren S, Handel SN (1999) Jumping spiders (Salticidae) enhance the seed production of a plant with extrafloral nectaries. Oecologia 119:227–230
Schmitz OJ, Hamback PA, Beckerman AP (2000) Trophic cascades in terrestrial ecosystems: a review of the effects of carnivore removals on plants. Am Nat 155:141–153
Shafir S, Roughgarden J (1998) Testing predictions of foraging theory for a sit-and-wait forager, Anolis gingivinus. Behav Ecol 9:74–84
Snyder WE, Wise DH (2001) Contrastic trophic cascades generated by a community of generalist predators. Ecology 82:1571–1583
Steffan-Dewenter I, Münzenberg U, Tscharntke T (2001) Pollination, seed set, and seed predation on a landscape scale. Proc R Soc Lond B 268:1685–1690
Suttle KB (2003) Pollinators as mediators of top-down effects on plants. Ecol Lett 6:688–694
Théry M, Casas J (2002) Predator and prey views of spider camouflage. Nature 415:133
Unsicker SB, Kunert G, Gershenzon J (2009) Protective perfumes: the role of vegetative volatiles in plant defense against herbivores. Curr Opin Plant Biol 12:479–485
Vet LEM, Dicke M (1992) Ecology of infochemical use by natural enemies in a tritrophic context. Annu Rev Entomol 37:141–172
Waites AR, Agren J (2004) Pollinator visitation, stigmatic pollen loads and among-population variation in seed set in Lythrum salicaria. J Ecol 92:512–526
Waser NM (1986) Flower constancy: definition, cause and measurement. Am Nat 127:593–603
Whitney KD (2004) Experimental evidence that both parties benefit in a facultative plant-spider mutualism. Ecology 85:1642–1650
Acknowledgments
The authors are grateful to the Institute of Neuroscience at Newcastle University for hosting A. D. H. and J. S. while this work was carried out. This manuscript was improved by the comments of three anonymous reviewers. A. D. H. was supported by Natural Environment Research Council (UK) grants NE/E016626/1 awarded to G. D. R. and NE/E018521/1 awarded to Mike Speed. J. S. was supported by NE/E016626/1 awarded to G. D. R. G. D. R. is also supported by NERC grants NE/F002653/1, NE/D010500/1 and NE/D010772/1. The authors declare that they have no conflict of interest and that this work complied with the current laws of the country in which it was carried out.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Judith Bronstein.
Rights and permissions
About this article
Cite this article
Higginson, A.D., Ruxton, G.D. & Skelhorn, J. The impact of flower-dwelling predators on host plant reproductive success. Oecologia 164, 411–421 (2010). https://doi.org/10.1007/s00442-010-1681-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-010-1681-6