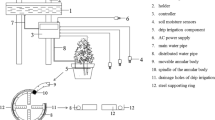
Abstract
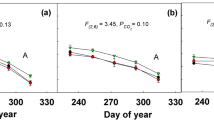
Long-term exposure to elevated CO2 concentration will affect the traits of wild plants in association with other environmental factors. We investigated multiple effects of atmospheric CO2 concentration, irradiance, and soil N availability on the leaf photosynthetic traits of a herbaceous species, Polygonum sachalinense, growing around natural CO2 springs in northern Japan. Atmospheric CO2 concentration and its interaction with irradiance and soil N availability affected several leaf traits. Leaf mass per unit area increased and N per mass decreased with increasing CO2 and irradiance. Leaf N per area increased with increasing soil N availability at higher CO2 concentrations. The photosynthetic rate under growth CO2 conditions increased with increasing irradiance and CO2, and with increasing soil N at higher CO2 concentrations. The maximal velocity of ribulose 1,5-bisphosphate carboxylation (V cmax) was affected by the interaction of CO2 and soil N, suggesting that down-regulation of photosynthesis at elevated CO2 was more evident at lower soil N availability. The ratio of the maximum rate of electron transport to V cmax (J max/V cmax) increased with increasing CO2, suggesting that the plants used N efficiently for photosynthesis at high CO2 concentrations by changes in N partitioning. To what extent elevated CO2 influenced plant traits depended on other environmental factors. As wild plants are subject to a wide range of light and nutrient availability, our results highlight the importance of these environmental factors when the effects of elevated CO2 on plants are evaluated.






Similar content being viewed by others
References
Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165:351–372
Ainsworth EA, Davey PA, Hymus GJ, Osborne CP, Rogers A, Blum H, Nösberger J, Long SP (2003) Is stimulation of leaf photosynthesis by elevated carbon dioxide concentration maintained in the long term? A test with Lolium perenne grown for 10 years at two nitrogen fertilization levels under free air CO2 enrichment (FACE). Plant Cell Environ 26:705–714
Badiani M, Raschi A, Paolacci AR, Miglietta F (2000) Plant responses to elevated CO2; a perspective from natural CO2 springs. In: Agrawal SB, Agrawal M (eds) Environmental pollution and plant responses. Lewis, Florida, pp 45–81
Bartak M, Raschi A, Tognetti R (1999) Photosynthetic characteristics of sun and shade leaves in the canopy of Arbutus unedo L. trees exposed to in situ long-term elevated CO2. Photosynthetica 37:1–16
Bettarini I, Calderoni G, Miglietta F, Raschi A, Ehleringer J (1995) Isotopic carbon discrimination and leaf nitrogen content of Erica arborea L. along a CO2 concentration gradient in a CO2 spring in Italy. Tree Physiol 15:327–332
Cook AC, Tissue DT, Roberts SW, Oechel WC (1998) Effects of long-term elevated CO2 from natural CO2 springs on Nardus stricta: photosynthesis, biochemistry, growth and phenology. Plant Cell Environ 21:417–425
Cotrufo MF, Raschi A, Lanini M, Ineson P (1999) Decomposition and nutrient dynamics of Quercus pubescens leaf litter in a naturally enriched CO2 Mediterranean ecosystem. Funct Ecol 13:343–351
Curtis PS, Wang X (1998) A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia 113:299–313
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 plants. Planta 149:78–90
Fernandez MD, Pieters A, Donoso C, Tezara W, Azkue M, Herrera C, Rengifo E, Herrera A (1998) Effects of a natural source of very high CO2 concentration on the leaf gas exchange, xylem water potential and stomatal characteristics of plants of Spatiphylum cannifolium and Bauhinia multinervia. New Phytol 138:689–697
Gahrooee FR (1998) Impacts of elevated atmospheric CO2 on litter quality, litter decomposability and nitrogen turnover rate of two oak species in a mediterranean forest ecosystem. Glob Chang Biol 4:667–677
Hikosaka K (2005) Nitrogen partitioning in the photosynthetic apparatus of Plantago asiatica leaves grown under different temperature and light conditions: similarities and differences between temperature and light acclimation. Plant Cell Physiol 46:1283–1290
Hikosaka K, Hirose T (1998) Leaf and canopy photosynthesis of C3 plants at elevated CO2 in relation to optimal partitioning of nitrogen among photosynthetic components: theoretical prediction. Ecol Model 106:247–259
Hikosaka K, Nagashima H, Harada Y, Hirose T (2001) A simple formulation of interaction between individuals competing for light in a monospecific stand. Funct Ecol 15:642–646
Hikosaka K, Ishikawa K, Borjigidai A, Muller O, Onoda Y (2006) Temperature acclimation of photosynthesis: mechanisms involved in the changes in temperature dependence of photosynthetic rate. J Exp Bot 57:291–302
Hogan KP, Smith AP, Ziska LH (1991) Potential effects of elevated CO2 and changes in temperature on tropical plants. Plant Cell Environ 14:763–778
Jones MB, Brown JC, Raschi A, Miglietta F (1995) The effects of Arbutus unedo L. of long-term exposure to elevated CO2. Glob Chang Biol 1:295–302
Keeney DR, Nelson DW (1982) Nitrogen-inorganic forms. In: Page AL (ed) Methods of soil analysis, part 2. American Society of Agronomy, Madison, pp 643–698
Knepp RG, Hamilton JG, Mohan JE, Zangerl AR, Berenbaum MR, DeLucia EH (2005) Elevated CO2 reduces leaf damage by insect herbivores in a forest community. New Phytol 167:207–218
Koch GW (1994) The use of natural situations of CO2 enrichment in studies of vegetation responses to increasing atmospheric CO2. In: Schulze ED, Mooney HA (eds) Design and execution of experiments on CO2 enrichment. Commission of the European Communities, Luxembourg, pp 318–392
Kohut R (2003) The long-term effects of carbon dioxide on natural systems: issues and research needs. Environ Int 29:171–180
Körner C (1995) Towards a better experimental basis for upscaling plant responses to elevated CO2 and climate warming. Plant Cell Environ 18:1101–1110
Körner C, Miglietta F (1994) Long term effects of naturally elevated CO2 on mediterranean grassland and forest trees. Oecologia 99:343–351
Long SP, Ainsworth EA, Rogers A, Ort DR (2004) Rising atmospheric carbon dioxide: plants FACE the future. Annu Rev Plant Biol 55:591–628
Luo Y, Reynolds J (1999) Validity of extrapolating field CO2 experiments to predict carbon sequestration in natural ecosystems. Ecology 80:1568–1583
Medlyn BE (1996) The optimal allocation of nitrogen within the C3 photosynthetic system at elevated CO2. Aust J Plant Physiol 23:593–603
Medlyn BE, Badeck FW, de Pury DGG, Barton CVM, Broadmeadow M, Ceulemans R, de Angelis P, Forstreuter M, Jach ME, Kellomaki S, Laitat E, Marek M, Philippot S, Rey A, Strassemeyer J, Laitinen K, Liozon R, Portier B, Roberntz P, Wang K, Jarvis PG (1999) Effects of elevated CO2 on photosynthesis in European forest species: a meta-analysis of model parameters. Plant Cell Environ 22:1475–1495
Miglietta F, Raschi A, Bettarini I, Resti R, Selvi F (1993) Natural CO2 springs in Italy: a resource for examining long-term response of vegetation to rising atmospheric CO2 concentrations. Plant Cell Environ 16:873–878
Miglietta F, Raschi A, Bettarini I, Badiani M, van Gardingen P (1994) Carbon dioxide springs and their use for experimentation. In: Schulze ED, Mooney HA (eds) Design and execution of experiments on CO2 enrichment. Commission of the European Communities, Luxembourg, pp 393–403
Newton PCD, Bell CC, Clark H (1996) Carbon dioxide emissions from mineral springs in Northland and the potential of these sites for studying the effects of elevated carbon dioxide on pastures. NZ J Agric Res 39:33–40
Niinemets Ü (1999) Components of leaf dry mass per area—thickness and density—alter leaf photosynthetic capacity in reverse directions in woody plants. New Phytol 144:35–47
Onoda Y, Hirose T, Hikosaka K (2007) Effect of elevated CO2 levels on leaf starch, nitrogen and photosynthesis of plants growing at three natural CO2 springs in Japan. Ecol Res 22:475–484
Onoda Y, Hirose T, Hikosaka K (2009) Does leaf photosynthesis adapt to CO2-enriched environments? An experiment on plants originating from three natural CO2 springs. New Phytol 182:698–709
Osada N, Takeda H, Furukawa A, Awang M (2001) Leaf dynamics and maintenance of tree crowns in a Malaysian rain forest stand. J Ecol 89:774–782
Pfanz H, Vodnik D, Wittmann C, Aschan G, Raschi A (2004) Plants and geothermal CO2 exhalations-survival in and adaptation to a high CO2 environment. Prog Bot 65:499–537
Piel C, Frak E, Le Roux X, Genty B (2002) Effect of local irradiance on CO2 transfer conductance of mesophyll in walnut. J Exp Bot 53:2423–2430
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophyll a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975:384–394
Prichard SG, Rogers HH, Prior SA, Peterson CM (1999) Elevated CO2 and plant structure: a review. Glob Chang Biol 5:807–837
Rogers A, Humphries SW (2000) A mechanistic evaluation of photosynthetic acclimation at elevated CO2. Glob Chang Biol 6:1005–1011
Roumet C, Laurent G, Roy J (1999) Leaf structure and chemical composition as affected by elevated CO2: genotypic responses of two perennial grasses. New Phytol 143:73–81
Sage RF (1994) Acclimation of photosynthesis to increasing atmospheric CO2: the gas exchange perspective. Photosynth Res 39:351–368
Sims DA, Seemann JR, Luo Y (1998) The significance of differences in the mechanisms of photosynthetic acclimation to light, nitrogen and CO2 for return on investment in leaves. Funct Ecol 12:185–194
Sokal RR, Rohlf FJ (1995) Biometry. Freeman, New York
Stitt M (1991) Rising CO2 levels and their potential significance for carbon flow in photosynthetic cells. Plant Cell Environ 14:741–762
Stylinski CD, Oechel WC, Gamon JA, Tissue DT, Miglietta F, Raschi A (2000) Effects of lifelong [CO2] enrichment on carboxylation and light utilization of Quercus pubescens willd. examined with gas exchange, biochemistry and optical techniques. Plant Cell Environ 23:1353–1362
Tognetti R, Johnson JD, Michelozzi M, Raschi A (1998) Response of foliar metabolism in mature trees of Quercus pubescens and Quercus ilex to long-term elevated CO2. Environ Exp Bot 39:233–245
Villar R, Held AA, Merino J (1995) Dark leaf respiration in light and darkness of an evergreen and a deciduous plant species. Plant Physiol 107:421–427
Vodnik D, Pfanz H, Macek I, Kastelec D, Lojen S, Batic F (2002) Photosynthesis of cockspur [Echinochloa crus-galli (L.) Beauv.] at sites of naturally elevated CO2 concentration. Photosynthetica 40:575–579
Von Caemmerer S, Evans JR, Hudson GS, Andrews TJ (1994) The kinetics of ribulose-1,5-bisphosphate carboxylase/oxygenase in vivo inferred from measurements of photosynthesis in leaves of transgenic tobacco. Planta 195:88–97
Ward JK, Strain BR (1999) Elevated CO2 studies: past, present and future. Tree Physiol 19:211–220
Webber AN, Nie GY, Long SP (1994) Acclimation of photosynthetic proteins to rising atmospheric CO2. Photosynth Res 39:413–425
Witkowski ETF, Lamong BB (1991) Leaf specific mass confounds leaf density and thickness. Oecologia 88:486–493
Yin X (2002) Responses of leaf nitrogen concentration and specific leaf area to atmospheric CO2 enrichment: a retrospective synthesis across 62 species. Glob Chang Biol 8:631–642
Acknowledgments
We thank the landowner (Hakkoda-Onsen, Tashiro Bokuya-Chikusan Kumiai) for permission to use the site for this study, Aki Shigeno for her help in field measurements, Riichi Oguchi for his help in anatomical analysis, and Onno Muller, Satoki Sakai, and Naoko Tokuchi for their valuable comments. This study was supported in part by grants from the Japan Ministry of Education, Culture, Sports, Science and Technology (18770011 and 21780140), and the Global Environment Research Fund (F-052) from the Japan Ministry of the Environment, and by the Sumitomo Foundation (073130).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Rowan Sage.
Rights and permissions
About this article
Cite this article
Osada, N., Onoda, Y. & Hikosaka, K. Effects of atmospheric CO2 concentration, irradiance, and soil nitrogen availability on leaf photosynthetic traits of Polygonum sachalinense around natural CO2 springs in northern Japan. Oecologia 164, 41–52 (2010). https://doi.org/10.1007/s00442-010-1635-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-010-1635-z