Abstract
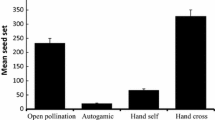
Although several factors can limit female fertility in perennial plants, rarely have they been jointly studied in a single species over several years. In this study we experimentally manipulate seed production and simultaneously analyse the potential contribution of pollen limitation, costs of reproduction and plant size to variation in seed output over a 3-year period in the perennial herb Paeonia officinalis, in southern France. Since this rare species is threatened by forest closure in many sites we also examine the causes of female fertility variation in relation to habitat closure (open habitat vs. woodland). P. officinalis has a partial self-incompatibility system and only very low ability for autonomous self pollination in the absence of pollinators. However, supplementary pollination of individual plants in three consecutive years did not significantly increase seed production above natural levels. Forest closure was associated with a decline in ovule and seed production, which again was not due to pollen limitation since supplementary pollination had no significant effect on seed set in the woodland habitat. Comparison of the maternal fertility of plants which were previously excluded from reproduction with those which were hand pollinated to maximise seed set in two previous years produced no evidence that seed production in year three is limited by costs associated with prior reproduction. Likewise, flowering probability was not related to prior seed production but was however positively related to plant size. The absence of any influence of pollen limitation or prior reproduction on seed production suggests that sub-maximal seed production in long-lived perennial herbs may be part of a size-dependent strategy that maximises life-time seed production and fitness without compromising survival.





Similar content being viewed by others
References
Ågren J, Willson MF (1994) Cost of seed production in the perennial herbs Geranium maculatum and G. sylvaticum—an experimental field-study. Oikos 70:35–42
Andrieu E, Debussche M, Thompson JD (2007) The impact of forest spread on a marginal population of a protected peony (Paeonia officinalis L.): the importance of conserving the habitat mosaic. Biodivers Conserv. doi:10.1007/s10531-005-2357-0
Ashman T-L (1992) Indirect costs of seed production within and between seasons in a gynodioecious species. Oecologia 92:266–272
Ashman T-L, Schoen DJ (1994) How long should flowers live? Nature 371:788–791
Ashman T-L, Schoen DJ (1996) Floral longevity: fitness consequences and resource costs. In: Lloyd DG, Barett SCH (eds) Floral biology. Studies on floral evolution in animal-pollinated plants. Chapman and Hall, New York, pp 112–139
Ashman TL, Knight TM, Steets JA, Amarasekare P, Burd M, Campbell DR, Dudash MR, Johnston MO, Mazer SJ, Mitchell RJ, Morgan MT, Wilson WG (2004) Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology 85:2408–2421
Baker AM, Barrett SCH, Thompson JD (2000) Variation of pollen limitation in the early flowering Mediterranean geophyte Narcissus assoanus (Amaryllidaceae). Oecologia 124:529–535
Barkham JP (1980) Population-dynamics of the Wild Daffodil (Narcissus pseudonarcissus). 1. Clonal growth, seed reproduction, mortality and the effects of density. J Ecol 68:607–633
Bierzychudek P (1981) Pollinator limitation of plant reproductive effort. Am Naturalist 117:838–840
Brunet J. (1996) Male reproductive success and variation in fruit and seed set in Aquilegia caerulea (Ranunculaceae). Ecology 77:2458–2471
Carmel Y, Kadmon R (1999) Effects of grazing and topography on long-term vegetation changes in a Mediterranean ecosystem in Israel. Plant Ecol 145:243–254
Charlesworth D (1989) Evolution of low female fertility in plants: pollen limitation, ressource allocation and genetic load. Trends Ecol Evolut 4:289–292
Charpentier A, Grillas P, Thompson JD (2000) The effects of population size limitation on fecundity in mosaic populations of the clonal macrophyte Scirpus maritimus. Am J Bot 87:502–507
Daget P (1977) Mediterranean bioclimate—general characteristics and modes of definition. Vegetatio 34:1–20
Debussche M, Lepart J, Dervieux A (1999) Mediterranean landscape change: evidence from old postcards. Global Ecol Biogeography 8:3–15
Ehrlén J (1992) Proximate limits to seed production in a herbaceous perennial legume, Lathyrus vernus. Ecology 73:1820–1831
Ehrlén J, Van Groenendael J (2001) Storage and the delayed costs of reproduction in the understorey perennial Lathyrus vernus. J Ecol 89:237–246
Elle E, Carney R (2003) Reproductive assurance varies with flower size in Collinsia parviflora (Scrophulariaceae). Am J Bot 90:888–896
Garcia D, Zamora R. (2003) Persistence, multiple demographic strategies and conservation in long-lived Mediterranean plants. J Veget Sci 14:921–926
Goverde M, Schweizer K, Baur B, Erhardt A (2002) Small-scale fragmentation effects on pollinator behaviour: experimental evidence from the bumblebee Bombus veteranus on calcareous grasslands. Biol Conserv 104:293–299
Griffin SR, Barret SCH (2002) Factors affecting low seed:ovule ratios in a spring woodland herb, Trillium grandiflorum (Melantiaceae). Int J Plant Sci 163:581–590
Haig D, Westoby M (1988) On limits to seed production. Am Natur 131:757–759
Herrera CM, Sanchez-Lafuente AM, Medrano M, Guitian J, Cerda X, Rey P (2001) Geographical variation in autonomous self-pollination levels unrelated to pollinator service in Helleborus foetidus (Ranunculaceae). Am J Bot 88:1025–1032
Husband BC, Schemske DW (1996) Evolution of the magnitude and timing of inbreeding depression in plants. Evolution 50:54–70
Johnson SD, Collin CL, Wissman HJ, Halvarsson E, Ågren J (2004) Factors contributing to variation in seed production among remnant populations of the endangered daisy Gerbera aurantiaca. Biotropica 36:148–155
Kreyer D, Oed A, Walther-Hellwig K, Frankl R (2004) Are forests potential landscape barriers for foraging bumblebees? Landscape scale experiments with Bombus terrestris agg. and Bombus pascuorum (Hymenoptera, Apidae). Biol Conserv 116:111–118
Knight TM, Steets JA, Vamosi JC, Mazer SJ, Burd M, Campbell DR, Dudash MR, Johnston MO, Mitchell RJ, Ashman TL (2005) Pollen limitation of plant reproduction: pattern and process. Ann Rev Ecol Evol Syst 36:467–497
Knight TM, Steets JA, Ashman TL. (2006) A quantitative synthesis of pollen supplementation experiments highlights the contribution of resource reallocation to estimates of pollen limitation. Am J Bot 93:271–277
Lavergne S, Thompson JD, Garnier E, Debussche M (2004) The biology and ecology of endemic and widespread plants: a comparative study of trait variation in 20 congeneric pairs. Oikos 107:505–518
Lavergne S, Debussche M, Thompson JD (2005) Limitations on reproductive success in endemic Aquilegia viscosa (Ranunculaceae) relative to its widespread congener Aquilegia vulgaris: the interplay of herbivory and pollination. Oecologia 142:212–220
Lawrence WS (1993) Resource and pollen limitation: plant size-dependent reproductive patterns in Physalis longifolia. Am Natur 141:296–313
Lee TD, Bazzaz FA (1982) Regulation of fruit and seed production in an annual legume, Cassia fasciculata. Ecology 63:1363–1373
Lloyd DG, Schoen DJ (1992) Self-and cross-fertilization in plants. I. Functional aspects. Int J Plant Sci 153:358–369
Morgan MT, Schoen DJ, Bataillon TM (1997) The evolution of self-fertilization in perennials. Am Natur 150:618–638
Ne’eman G (2003) To be or not to be—the effect of nature conservation management on flowering of Paeonia mascula (L.) Miller in Israel. Biol Conserv 109:103–109
Obeso JR (2002) The costs of reproduction in plants. New Phytol 155:321–348
Pino J, Sans FX, Masalles RM (2002) Size-dependent reproductive pattern and short-term reproductive cost in Rumex obtusifolius L. Acta Oecol 23:321–328
Primack RB, Hall P (1990) Costs of reproduction in the Pink Lady’s Slipper Orchid—a 4-year experimental study. Am Natur 136:638–656
Rodriguez-Perez J. (2005) Breeding system, flower visitors and seedling survival of two endangered species of Helianthemum (Cistaceae). Ann Bot 95:1229–1236
Sanchez-Lafuente AM, Rey PJ, Alcantara JM, Valera F (1999) Breeding system and the role of floral visitors in seed production of a “few-flowered” perennial herb, Paeonia broteroi Boiss. & Reut. (Paeoniaceae). Ecoscience 6:163–172
SAS Institute Inc. (1999) SAS/STAT user’s guide. Cary
Siemens DH, Johnson CD (1995) Number of seeds per pod in 3 species of perennial legumes—a compromise between ecological and physiological constraints. Am Midland Natur 133:197–205
Silvertown J, Franco M, Pisanty I, Mendoza A (1993) Comparative plant demography-relative importance of life-cycle components to the finite rate of increase in woody and herbaceous perennials. J Ecol 81:465–476
StatSoft France (2005) Statistica
Steffan-Dewenter I, Tscharntke T (1999) Effects of habitat isolation on pollinator communities and seed set. Oecologia 121:432–440
Stephenson AG (1981) Flower and fruit abortion: proximate causes and ultimate functions. Ann Rev Ecol Syst 12:253–279
Sutherland S (1986) Patterns of fruit set: what controls fruit-flower ratios in plants. Evolution 40:117–128
Sutherland S, Delph LF (1984) On the importance of male fitness in plants: patterns of fruit set. Ecology 65:1093–1104
Thompson JD (2005) Plant evolution in the Mediterranean. Oxford University Press, Oxford
Thompson JD, Dommée B (2000) Morph specific stigma height variation in distylous Jasminum fruticans. New Phytol 148:303–314
Travis J (1992) Components of reproductive success in the herbaceous perennial Amianthum muscaetoxicum. In: Wyatt R (ed) Ecology and evolution of plant reproduction. Chapman and Hall, New York, pp 255–280
Tutin TG, Burges NA, Chater AO, Edmondson JR, Heywood VH, Moore DM, Valentine DH, Walters SM, Webb DA (1993) Flora Europaea. Cambridge University Press, Cambridge
Valverde T, Silvertown J (1995) Spatial variation in the seed ecology of a woodland herb (Primula vulgaris) in relation to light environment. Funct Ecol 9:942–950
Wiens D (1984) Ovule survivorship, brood size, life history, breeding systems, and reproductive success in plants. Oecologia 64:47–53
Williams EG, Knox RB (1982) Quantitative analysis of pollen-tube growth in Lycopersicon peruvianum. J Palynol 18:65–74
Worley AC, Harder LD (1996) Size-dependent resource allocation and costs of reproduction in Pinguicula vulgaris (Lentibulariaceae). J Ecol 84:195–206
Acknowledgments
This work was funded by the European programme “Mediterranean Mountain Ecosystems in a Changing World”, the C.N.R.S Long-Term Research Area “Mediterranean Backcountry”, grant ANR-05-BDIV-014 form the Agence Nationale de la Recherche, a Ph.D grant from the French government to EA and a Marco Polo research grant from AlmaUE (University of Bologna) to MG. We are grateful to Adeline André, Laure Andrieu, Audrey Coreau and Anabelle Dossantos for help with field work and Mr and Mrs Buresi and the inhabitants of Vissec for access to private land. All experiments in this study complied with the current French law concerning protected species.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Russell Monson.
Rights and permissions
About this article
Cite this article
Andrieu, E., Debussche, M., Galloni, M. et al. The interplay of pollination, costs of reproduction and plant size in maternal fertility limitation in perennial Paeonia officinalis . Oecologia 152, 515–524 (2007). https://doi.org/10.1007/s00442-007-0662-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-007-0662-x