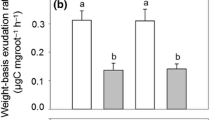
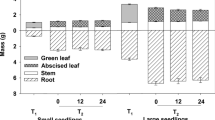
Abstract
Nitrogen uptake, nitrogen demand and internal nitrogen cycling were studied to address the question of the importance of nutrient storage in Quercus species with contrasting leaf longevities. We carried out this study at the whole-plant level with young trees (2–4 years old) of three Mediterranean Quercus species: the evergreen Q. ilex, the marcescent/evergreen Q. faginea, and the deciduous Q. pyrenaica. Seasonal dynamics of nitrogen in all compartments of the plant were followed for 3 years. Nitrogen losses were measured through litter production, herbivory and fine root shedding. Nitrogen uptake was estimated using increments of nitrogen plant content plus accumulative nitrogen losses. Nitrogen uptake was limited to a few months during late winter and spring. Before budbreak, acquired nitrogen was stored in old-leaf cohorts of evergreen and woody compartments. After budbreak, Quercus species relied first on soil uptake and second on nitrogen retranslocation to supply new growth requirements. However, in most cases we found a high asynchrony between nitrogen demand by growing tissues and soil supply, which determined a strong nitrogen retranslocation up to 88.4% of the nitrogen demand throughout leaf expansion. Except for the first year after planting, the above- and underground woody fractions provided more nitrogen to the new tissues than the old leaf cohorts. Differences in the benefit of nitrogen withdrawn from senescent and old leaves were not found between species. We conclude that sink/source interaction strength was determined by differences between nitrogen demand and uptake, regulating internal nutrient cycling at the whole plant level.







Similar content being viewed by others
References
Aerts R (1989) Aboveground biomass and nutrient dynamics of Calluna vulgaris and Molinia caerulea in a dry heathland. Oikos 56:31–38
Aerts R (1993) Biomass and nutrient dynamics of dominant plant species in heathlands. In: Aerts R, Heil GW (eds) Heathlands, patterns and processes in a changing environment. Kluwer Academic, Dordrecht, pp 51–84
Aerts R (1996) Nutrient resorption from senescing leaves of perennials: are there general patterns? J Ecol 84:597–608
Aerts R, Chapin FS (2000) The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv Ecol Res 30:1–67
Berendse F, Elberse WT (1990) Competition and nutrient availability in heathland and grassland ecosystems. In: Grace JB, Tilman D (eds) Perspectives on plant competition. Academic Press, New York, pp 93–116
Berendse F, de Kroon H, Braakhekke WG (1999) Acquisition, use and loss of nutrients. In: Pugnaire FI, Valladares F (eds) Handbook of functional ecology. Dekker, New York, pp 315–345
Bilbrough CJ, Caldwell MM (1997) Exploitation of springtime ephemeral N pulses by six Great basin plant species. Ecology 78:231–243
Bloom AJ, Chapin FS, Mooney HA (1985) Resource limitation in plants- an economic analogy. Annu Rev Ecol Syst 16:363–392
Campbell BD, Grime JP (1992) An experimental test of plant strategy theory. Ecology 73:15–29
Canadell J, Zedler PH (1995) Underground structures of woody plants in mediterranean ecosystems of Australia, California, and Chile. In: Arroyo MKT, Zedler PH, Fox MD (eds) Ecology and biogeography of mediterranean ecosystems in Chile, California, and Australia. Ecological Studies 108. Springer, Berlin Heidelberg New York, pp 177–210
Chabot BF, Hicks DJ (1982) The ecology of leaf life spans. Annu Rev Ecol Syst 13:229–259
Chapin FS (1980) The mineral nutrition of wild plants. Annu Rev Ecol Syst 11:233–260
Chapin FS (1991) Effects of multiple environmental stresses on nutrient availability and use. In: Mooney HA, Winner WE, Pell EJ (eds) Response of plants to multiple stresses. Academic Press, pp 67–88
Chapin FS, Moilanen L (1991) Nutritional controls over nitrogen and phosphorus resorption from Alaskan birch leaves. Ecology 72:709–715
Chapin FS, Shaver GR (1989) Differences in growth and nutrient use among artic plant growth forms. Funct Ecol 3:73–80
Chapin FS, Schulze E, Mooney HA (1990) The ecology and economics of storage in plants. Annu Rev Ecol Syst 21:423–447
Dixon KR (1976) Analysis of seasonal leaf fall in north temperate deciduous forests. Oikos 27:300–306
Dyckmans J, Flessa H (2002) Influence of tree internal N status on the reactions of beech (Fagus sylvatica L.) to elevated atmospheric CO2. Tree Physiol 22:41–49
Dyckmans J, Flessa H, Brinkmann K, Mai C, Polle A (2002) Carbon and nitrogen dynamics in acid detergent fibre lignins of beech (Fagus sylvatica L.) during the growth phase. Plant Cell Environ 25:469–478
Eckstein RL, Karlsson PS, Weih M (1998) The significance of resorption of leaf resources for shoot growth in evergreen and deciduous woody plants from a subarctic environment. Oikos 81:567–575
Eissenstat DM, Yanai RD (1997) The ecology of root lifespan. Adv Ecol Res 27:1–60
Escudero A, del Arco JM, Garrido MV (1992) The efficiency of nitrogen retranslocation from leaf biomass in Quercus ilex ecosystems. Vegetatio 99–100:225–237
Evans RD, Ehleringer JR (1994) Water and nitrogen dynamics in an arid woodland. Oecologia 99:233–242
Gleeson SK, Tilman D (1990) Allocation and the transient dynamics of succession on poor soils. Ecology 71:1144–1155
Greenway KJ, Macdonald SE, Lieffers V (1992) Is long-lived foliage in Picea mariana an adaptation to nutrient-poor conditions? Oecologia 91:184–191
Heilmeier H, Monson RK (1994) Carbon and nitrogen storage in herbaceous plants. In: Roy J, Garnier E (eds) A whole plant perspective on carbon- nitrogen interactions. SPB Academic, The Hague, pp 149–172
Heilmeier H, Schulze ED, Whale DM (1986) Carbon and nitrogen partitioning in the biennial monocarp Arcticum tomentosum Mill. Oecologia 70:466–474
Jaeger CH, Monson RK (1992) Adaptive significance of nitrogen storage in Bistorta bistortoides, an alpine herb. Oecologia 92:578–585
Jaerger CH, Monson RK, Fisk MC, Schmidt SK (1999) Seasonal partitioning of nitrogen by plants and soil microorganisms in an alpine ecosystem. Ecology 80:1883–1892
Jonasson S (1989) Implications of leaf longevity, leaf nutrient re-absorption and translocation for the resource economy of five evergreen plant species. Oikos 56:121–131
Jonasson S (1995a) Resource allocation in relation to leaf retention time of the wintergreen Rhododendron lapponicum. Ecology 76:475–485
Jonasson S (1995b) Nutrient limitation in Rhododendron lapponicum, fact or artefact? Oikos 73:269–273
Jonasson S, Chapin FS (1985) Significance of sequential leaf development for nutrient balance of the cotton sedge, Eriophorum vaginatum L. Oecologia 67:511–518
Karlsson PS (1994) The significance of internal nutrient cycling in branches for growth and reproduction of Rhododendron lapponicum. Oikos 70:191–200
Karlsson PS (1995) Nutrient or carbon limitation of shoot growth in Rhododendron lapponicum- both or neither! A reply to Jonasson. Oikos 73:272–273
Killingbeck KT (1996) Nutrient in senesced leaves: keys to the search for potential resorption and resorption proficiency. Ecology 77:1716–1727
Kruger EL, Reich PB (1997) Responses of hardwood regeneration to fire in mesic forest openings. III. Whole-plant growth, biomass distribution, and nitrogen and carbohydrate relations. Can J For Res 27:1841–1850
Lambers H, Chapin FS, Pons TL (1998) Plant physiological ecology. Springer, Berlin Heidelberg New York
Layzell DB (1990) N2 fixation, NO3 - reduction and NH4 + assimilation. In: Dennis DT, Turpin DH (eds) Plant physiology, biochemistry and molecular biology. Longman, New York, pp 389–406
Lodge DJ, McDowell WH, McSwiney CP (1994) The importance of nutrient pulses in tropical forests. Trends Ecol Evol 9:384–387
May JD, Killingbeck KT (1992) Effects of preventing nutrient resorption on plant fitness and foliar nutrient dynamics. Ecology 73:1868–1878
Mediavilla S (2000) Intercambios gaseosos en especies leñosas mediterráneas. Efectos de la longevidad y de otros rasgos foliares sobre la eficiencia fotosintética en el empleo del agua y del nitrógeno. PhD-Thesis, University of Salamanca
Nambiar EKS (1987) Do nutrient retranslocate from fine roots? Can J For Res 17:913–918
Nambiar EKS, Fife DN (1991) Nutrient retranslocation in temperate conifers. Tree Physiol 9:185–207
Nye PH, Tinker PB (1977) Solute movement in the soil-root system. University of California Press, Berkley
Poorter H (1994) Construction costs and payback time of biomass: a whole plant perspective. In: Roy J, Garnier E (eds) A whole plant perspective on carbon- nitrogen interactions. SPB Academic, The Hague, pp 111–129
Reader RJ (1978) Contribution of overwinter leaves to the growth of three broad-leaved, evergreen shrubs belonging to the Ericaceae family. Can J Bot 56:1248–1261
Reich PB (1998) Variation among plant species in leaf turnover rates and associated traits: implications for growth at all life stages. In: Lambers H, Poorter H, Van Vuuren MMI (eds) Inherent variation in plant growth. Physiological mechanisms and ecological consequences. Backhuys, Leiden, pp 467–487
Shaver GR, Chapin FS (1991) Production: biomass relationships and element cycling in contrasting artic vegetation types. Ecol Monogr 61:1–31
Shaver GR, Kummerow J (1992) Phenology, resource allocation, and growth of artic vascular plants. In: Chapin FS, Jeffries RL, Reynolds JF, Shaver GR, Svoboda J, Chu EW (eds) Arctic ecosystems in a changing climate. Academic Press, New York, pp 193–211
Silla F (2001) Producción y ciclo de nutrientes en reforestaciones de quercíneas. PhD-Thesis, University of Salamanca
Sokal RR, Rohlf FJ (1995) Biometry. Freeman, New York
Acknowledgements
F.S. thanks Rien Aerts for his valuable comments during his stay at Vrije Universitait. This work has received financial support from the Spanish Ministry of Education (Projects Nº FOR89-0845 and AMB95-0800) and from the Junta de Castilla y León (projects Nº SA47/95 and SA72/00B). We are grateful to two anonymous reviewers for their helpful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silla, F., Escudero, A. Uptake, demand and internal cycling of nitrogen in saplings of Mediterranean Quercus species. Oecologia 136, 28–36 (2003). https://doi.org/10.1007/s00442-003-1232-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-003-1232-5