Abstract
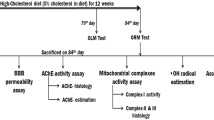
Hyperlipidemia (HLP) is one of the risk factors for memory impairment and cognitive impairment. However, its pathological molecular mechanism remained unclear. 3β-hydroxysterol Δ24- reductase (DHCR24) is a key enzyme in cholesterol synthesis and has been reported to decrease in the affected areas in the brain of neurodegenerative disorders. In this study, hyperlipidemic mouse model was established to study the effect of high blood lipid on brain. The data obtained from HPLC analysis demonstrated that the cholesterol level in the brain of mice with hyperlipidemia was significantly elevated compared to the control group. While the pathological damages were observed in both cerebral cortex and hippocampus in the brain of hyperlipidemic mice. Furthermore, the protein level of DHCR24 was downregulated accompanied by elevated ubiquitination level in the hyperlipidemic mice brain. The mouse neuroblastoma cells N2a were exposed to the excess cholesterol loading, the cells underwent apoptosis and the mRNA and protein of DHCR24 in cholesterol-loaded N2a cells were significantly reduced. In addition, the expression level of endoplasmic reticulum stress marker protein (Bip and Chop) was markedly increased in response to the cholesterol loading. More importantly, overexpression of DHCR24 in N2a reversed neuronal apoptosis induced by the cholesterol loading. Conclusively, these findings suggested that hyperlipidemia could cause brain tissue injuries via down-regulating DHCR24, and overexpression of DHCR24 may alleviate hyperlipidemia-induced neuronal cells damage by reversing the endoplasmic reticulum stress-mediated apoptosis.








Similar content being viewed by others
Data availability
Please contact author for data requests.
References
Andersson HC, Kratz L, Kelley R (2002) Desmosterolosis presenting with multiple congenital anomalies and profound developmental delay. Am J Med Genet 4:315–319
Bandosz P, Ahmadi-Abhari S, Guzman-Castillo M, Pearson-Stuttard J, Collins B, Whittaker H, Shipley MJ, Capewell S, Brunner EJ, O’Flaherty M (2020) Potential impact of diabetes prevention on mortality and future burden of dementia and disability: a modelling study. Diabetologia 63:104–115
Cecchi C, Rosati F, Pensalfini A, Formigli L, Nosi D, Liguri G et al (2008) Seladin-1/DHCR24 protects neuroblastoma cells against Abeta toxicity by increasing membrane cholesterol content. J Cell Mol Med 12:1990–2002
Chaowei Wu, Miloslavskaya I, Demontis S, Maestro R, Galaktionov K (2004) Regulation of cellular response to oncogenic and oxidative stress by Seladin-1. Nature 432:640–645
Chaudhari N, Talwar P, Parimisetty A et al (2014) A molecular web: endoplasmic reticulum stress, inflammation, and oxidative stress. Front Cell Neurosci 8:213
Chen C, Zissimopoulos JM (2018) Racial and ethnic differences in trends in dementia prevalence and risk factors in the United States. Alzheimer’s & Dementia: Translational Research & Clinical Interventions 4:510–520
Cybulsky AV (2013) The intersecting roles of endoplasmic reticulum stress, ubiquitin-proteasome system, and autophagy in the pathogenesis of proteinuric kidney disease. Kidney Int 84(1):25–33
Daimiel L, Fernández-Suárez M, Rodríguez-Acebes S (2012) Promoter analysis of the DHCR24 (3β-hydroxysterol Δ(24)-reductase) gene: characterization of SREBP (sterol-regulatory-element-binding protein)-mediated activation. Biosci Rep 33(1):57–69
de Oliveira J, Engel DF, de Paula GC, Dos Santos DB, Lopes JB et al (2020) High cholesterol diet exacerbates blood-brain barrier disruption in LDLr-/- mice: impact on cognitive function. J Alzheimers Dis 78(1):97–115
Dietschy JM, Turley SD (2001) Linking lipids to Alzheimer’s disease: cholesterol and beyond. Nat Rev Neurosci 12:284–296
Greeve I, Hermans-Borgmeyer I, Brellinger C, Kasper D, GomezIsla T (2000a) The human DIMINUTO/DWARF1 homolog seladin-1 confers resistance to Alzheimer’s disease-associated neurodegeneration and oxidative stress. J Neurosci 20(19):7345–7352
Gill S, Stevenson J, Kristiana I, Brown AJ (2011) Cholesterol-dependent degradation of squalene monooxygenase, a control point in cholesterol synthesis beyond HMG-CoA reductase. Cell Metab 13:260–273
Greeve I, Hermans-Borgmeyer I, Brellinger C, Kasper D, Gomez-Isla T et al (2000b) The human DIMINUTO/DWARF1 homolog seladin-1 confers resistance to Alzheimer’s disease-associated neurodegeneration and oxidative stress. J Neurosci 20:7345–7352
Iivonen S, Hiltunen M, Alafuzoff I, Mannermaa A, Kerokoski P (2002) Seladin-1 transcription is linked to neuronal degeneration in Alzheimer’s disease. Neuroscience 113:301–310
Ishfaq M, Zhang W, Wanying Hu, Shah SWA, Liu Y, Wang J et al (2019) Antagonistic effects of Baicalin on mycoplasma gallisepticum-induced inflammation and apoptosis by restoring energy metabolism in the chicken lungs. Infection and Drug Resistance 12:3075–3089
Jansen M, Wang W, Greco D, Bellenchi GC, di Porzio U et al (2013) What dictates the accumulation of desmosterol in the developing brain? FASEB J 27:865–870
Kacher R, Lamazie`re A, Heck N, Kappes V, Mounier C, et al (2019) CYP46A1 gene therapy deciphers the role of brain cholesterol metabolism in Huntington’s disease. Brain 142(8):2432–2450
Lämsä R, Helisalmi S, Hiltunen M, Herukka S-K, Tapiola T, Pirttilä T (2007) The association study between DHCR24 polymorphisms and Alzheimer’s disease. Am J Med Genet B Neuropsychiatr Genet 144B:906–910
Loera-Valencia R, Goikolea J, Parrado-Fernandez C, Merino-Serrais P, Maioli S (2019) Alterations in cholesterol metabolism as a risk factor for developing Alzheimer’s disease: Potential novel targets for treatment. J Steroid Biochem Mol Biol 190:104–114
Lourida I, Eilis Hannon, Thomas J Littlejohns et al (2019) Association of lifestyle and genetic risk with incidence of dementia. JAMA 14
Liu Y, Li Z, Song Y, Liu Y, Zhao H, Liu Y et al (2019) Study on urine metabolic profiling and pathogenesis of hyperlipidemia. Clin Chim Acta 495:365–373
Lu X, Li Y, Wang W, Chen S, Liu T (2014) 3 β-hydroxysteroid-Δ 24 reductase (DHCR24) protects neuronal cells from apoptotic cell death induced by endoplasmic reticulum (ER) stress. PloS One 9(1)
Martiskainen H, Paldanius KMA, Natunen T, Takalo M, Marttinen M, Leskela S et al (2017) DHCR24 exerts neuroprotection upon inflammation-induced neuronal death. J Neuroinflammation 14(1):215
Matthews FE, Stephan BC, Robinson L et al (2016) A two-decade dementia incidence comparison from the cognitive function and ageing studies I and II. Nat Commu 7(1):1–8
Maulik M, Westaway D, Jhamandas JH, Kar S (2013) Role of cholesterol in APP metabolism and its significance in Alzheimer’s disease pathogenesis. Mol Neurobiol 47:37–63
Organization WH (2019) Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines in Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines.
Paul R, Borah A (2017) Global loss of acetylcholinesterase activity with mitochondrial complexes inhibition and inflammation in brain of hypercholesterolemic mice. Sci Rep 7(1):17922
Schilling S, Tzourio C, Soumare A et al (2017) Differential associations of plasma lipids with incident dementia and dementia subtypes in the 3C study: a longitudinal, population-based prospective cohort study. PLoS Med 14(3):e1002265
Song YF, Liu JJ, Zhao K, Gao L, Zhao JJ (2021) Cholesterol-induced toxicity: An integrated view of the role of cholesterol in multiple diseases. Cell Metab 5 33(10):1911–1925
Wang H, Ziyin Lu, Li Y, Liu T, Zhao L, Gao T, Xiuli Lu, Gao B (2023) Virtual Screening of Novel 24-Dehydroxysterol Reductase (DHCR24) Inhibitors and the Biological Evaluation of Irbesartan in Cholesterol-Lowering Effect. Molecure 28(6):2643
Xiong J, Deng I, Kelliny S, LinL BL, Zhou XF (2022) Long term high fat diet induces metabolic disorders and aggravates behavioral disorders and cognitive defcits in MAPT P301L transgenic mice. Metab Brain Dis 37:1941–1957
Zerenturk EJ, Kristiana I, Gill S, Brown AJ (2012) The endogenous regulator 24(S), 25- epoxycholesterol inhibits cholesterol synthesis at DHCR24 (Seladin-1). Biochim Biophys Acta 1821:1269–1277
Zerenturk EJ, Sharpe LJ, Ikonen E, Brown AJ (2013) Desmosterol and DHCR24: unexpected new directions for a terminal step in cholesterol synthesis. Prog Lipid Res 52(4): 666-680
Zhou X, Zhang W, Liu X, Zhang W, Li Y (2015) Interrelationship between diabetes and periodontitis: role of hyperlipidemia. Arch Oral Biol 60(4):667-674
Acknowledgements
The authors greatly acknowledge the funding support for this study. In addition, we thank Dr. Muhammad Ishfaq (Huanggang Normal University) for his help in revising and improving the language expression in the manuscript.
Funding
The study was supported by a grant of National Natural Science Foundation of China (No. 31271494) and Liaoning provincial key R & D project (No. 2020JH2/10300114 and 2019JH8/10300057).
Author information
Authors and Affiliations
Contributions
Xiuli Lu and Bing Gao supervised the whole experiments. Ziyin Lu and Haozhen Wang, designed this study and contributed to the paper writing. Xiujin Zhang, Xiuting Huang, Shan Jiang, Chen Lu, Yang Li, and Ting Liu performed the practical work and completed the experiments. All authors have read and approved the final version of this manuscript to be published.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The present study was conducted in accordance with Laboratory Animal-Guideline for ethical review of animal welfare (GB/T 35,892–2018, National Standards of the People’s Republic of China).
Consent for publication
Not applicable.
Competing interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, Z., Wang, H., Zhang, X. et al. High fat diet induces brain injury and neuronal apoptosis via down-regulating 3-β hydroxycholesterol 24 reductase (DHCR24). Cell Tissue Res 393, 471–487 (2023). https://doi.org/10.1007/s00441-023-03804-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-023-03804-3