Abstract
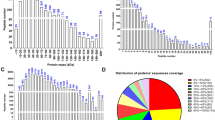
Asthenozoospermia, characterized by low sperm motility, is one of the most common causes of male infertility. While many intrinsic and extrinsic factors are involved in the etiology of asthenozoospermia, the molecular basis of this condition remains unclear. Since sperm motility results from a complex flagellar structure, an in-depth proteomic analysis of the sperm tail can uncover mechanisms underlying asthenozoospermia. This study quantified the proteomic profile of 40 asthenozoospermic sperm tails and 40 controls using TMT-LC–MS/MS. Overall, 2140 proteins were identified and quantified where 156 proteins have not been described earlier in sperm tail. There were 409 differentially expressed proteins (250 upregulated and 159 downregulated) in asthenozoospermia which by far is the highest number reported earlier. Further, bioinformatics analysis revealed several biological processes, including mitochondrial-related energy production, oxidative phosphorylation (OXPHOS), citric acid cycle (CAC), cytoskeleton, stress response, and protein metabolism altered in asthenozoospermic sperm tail samples. Collectively, our findings reveal the importance of mitochondrial energy production and induced stress response as potential mechanisms involved in the loss of sperm motility in asthenozoospermia.









Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
References
Agarwal A, Bertolla RP, Samanta L (2016) Sperm proteomics: potential impact on male infertility treatment. Expert Rev Proteomics 13:285–296
Amaral A, Castillo J, Estanyol J, Lagarda JL, Ramalho-Santos J, Oliva R (2012) Human sperm tail proteome suggests new endogenous metabolic pathways. Mol Cell Proteomics 12
Amaral A, Castillo J, Ramalho-Santos J, Oliva R (2014a) The combined human sperm proteome: cellular pathways and implications for basic and clinical science. Hum Reprod Update 20:40–62
Amaral A, Paiva C, Attardo Parrinello C, Estanyol JM, Ballescà JL, Ramalho-Santos J, Oliva R (2014b) Identification of proteins involved in human sperm motility using high-throughput differential proteomics. J Proteome Res 13:5670–5684
Amaral A, Paiva C, Baptista M, Sousa AP, Ramalho-Santos J (2011) Exogenous glucose improves long-standing human sperm motility, viability, and mitochondrial function. Fertil Steril 96:848–850
Amaral A, Ramalho-Santos J (2010) Assessment of mitochondrial potential: implications for the correct monitoring of human sperm function. Int J Androl 33:e180–e186
Ariumi Y (2014) Multiple functions of DDX3 RNA helicase in gene regulation, tumorigenesis, and viral infection. Front Genet 5:423
Asghari A, Marashi S-A, Ansari-Pour N (2017) A sperm-specific proteome-scale metabolic network model identifies non-glycolytic genes for energy deficiency in asthenozoospermia. Syst Biol Reprod Med 63:100–112
Baker MA, Naumovski N, Hetherington L, Weinberg A, Velkov T, Aitken RJ (2013) Head and flagella subcompartmental proteomic analysis of human spermatozoa. Proteomics 13:61–74
Bansal SK, Gupta N, Sankhwar SN, Rajender S (2015) Differential genes expression between fertile and infertile spermatozoa revealed by transcriptome analysis. PloS One 10
Brewis IA, Brennan P (2010) Proteomics technologies for the global identification and quantification of proteins. Advances in protein chemistry and structural biology, vol 80. Elsevier, pp 1–44
Budanov AV, Sablina AA, Feinstein E, Koonin EV, Chumakov PM (2004) Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science 304:596–600
Byagowi S, Naserpour Farivar T, Najafipour R, Sahmani M, Darabi M, Fayezi S, Mirshahvaladi S, Darabi M (2015) Effect of PPARδ agonist on stearoyl-CoA desaturase 1 in human pancreatic cancer cells: role of MEK/ERK1/2 pathway. Can J Diabetes 39:123–127
Cao X, Cui Y, Zhang X, Lou J, Zhou J, Bei H, Wei R (2018) Proteomic profile of human spermatozoa in healthy and asthenozoospermic individuals. Reprod Biol Endocrinol 16:16
Chan C-C, Shui H-A, Wu C-H, Wang C-Y, Sun G-H, Chen H-M, Wu G-J (2009) Motility and protein phosphorylation in healthy and asthenozoospermic sperm. J Proteome Res 8:5382–5386
Chandramouli K, Qian P-Y (2009) Proteomics: challenges, techniques and possibilities to overcome biological sample complexity. Hum Genomics Proteomics HGP 2009
Conway JR, Lex A, Gehlenborg N (2017) UpSetR: an R package for the visualization of intersecting sets and their properties. Bioinformatics 33:2938–2940
Curi S, Ariagno J, Chenlo P, Mendeluk G, Pugliese M, Sardi Segovia L, Repetto H, Blanco A (2003) asthenozoospermia: analysis of a large population. Arch Androl 49:343–349
Deng L, Pushpitha K, Joseph C, Gupta V, Rajput R, Chitranshi N, Dheer Y, Amirkhani A, Kamath K, Pascovici D, Wu JX, Salekdeh GH, Haynes PA, Graham SL, Gupta VK, Mirzaei M (2019) Amyloid β induces early changes in the ribosomal machinery, cytoskeletal organization and oxidative phosphorylation in retinal photoreceptor cells. Front Mol Neurosci 12:24
Ditton H, Zimmer J, Kamp C, Rajpert-De Meyts E, Vogt P (2004) The AZFa gene DBY (DDX3Y) is widely transcribed but the protein is limited to the male germ cells by translation control. Hum Mol Genet 13:2333–2341
Doncheva NT, Morris JH, Gorodkin J, Jensen LJ (2018) Cytoscape StringApp: network analysis and visualization of proteomics data. J Proteome Res 18:623–632
du Plessis SS, Kashou AH, Benjamin DJ, Yadav SP, Agarwal A (2011) Proteomics: a subcellular look at spermatozoa. Reprod Biol Endocrinol 9:36–36
Du Y, Li M, Chen J, Duan Y, Wang X, Qiu Y, Cai Z, Gui Y, Jiang H (2016) Promoter targeted bisulfite sequencing reveals DNA methylation profiles associated with low sperm motility in asthenozoospermia. Hum Reprod 31:24–33
Ergur AR, Dokras A, Giraldo JL, Habana A, Kovanci E, Huszar G (2002) Sperm maturity and treatment choice of in vitro fertilization (IVF) or intracytoplasmic sperm injection: diminished sperm HspA2 chaperone levels predict IVF failure. Fertil Steril 77:910–918
Folgerø T, Bertheussen K, Lindal S, Torbergsen T, Øian P (1993) Andrology: mitochondrial disease and reduced sperm motility. Hum Reprod 8:1863–1868
Garrido C, Solary E (2003) A role of HSPs in apoptosis through “protein triage”? Nature Publishing Group
GeneCards (2022) Gene Cards – the human gene database
Gilany K, Lakpour N, Vafakhah M, Sadeghi MR (2011) The profile of human sperm proteome; a mini-review. J Reprod Infertil 12:193–199
Gòdia M, Swanson G, Krawetz SA (2018) A history of why fathers’ RNA matters. Biol Reprod 99
Gu Z, Eils R, Schlesner M (2016) Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32:2847–2849
Guo Y, Jiang W, Yu W, Niu X, Liu F, Zhou T, Zhang H, Li Y, Zhu H, Zhou Z (2019) Proteomics analysis of asthenozoospermia and identification of glucose-6-phosphate isomerase as an important enzyme for sperm motility. J Proteomics 208:103478
Gur Y, Breitbart H (2006) Mammalian sperm translate nuclear-encoded proteins by mitochondrial-type ribosomes. Genes Dev 20:411–416
Gur Y, Breitbart H (2008) Protein synthesis in sperm: dialog between mitochondria and cytoplasm. Mol Cell Endocrinol 282:45–55
Hashemitabar M, Sabbagh S, Orazizadeh M, Ghadiri A, Bahmanzadeh M (2015) A proteomic analysis on human sperm tail: comparison between normozoospermia and asthenozoospermia. J Assist Reprod Genet 32:853–863
Inaba K, Mizuno K (2016) Sperm dysfunction and ciliopathy. Reprod Med Biol 15:77–94
Jodar M, Selvaraju S, Sendler E, Diamond M, Krawetz S, Network RM (2013) The presence, role and clinical use of spermatozoal RNAs. Hum Reprod Update 19
Khelifa MB, Coutton C, Zouari R, Karaouzène T, Rendu J, Bidart M, Yassine S, Pierre V, Delaroche J, Hennebicq S (2014) Mutations in DNAH1, which encodes an inner arm heavy chain dynein, lead to male infertility from multiple morphological abnormalities of the sperm flagella. Am J Hum Genet 94:95–104
Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, Koplev S, Jenkins SL, Jagodnik KM, Lachmann A, McDermott MG, Monteiro CD, Gundersen GW, Ma’ayan A (2016) Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res 44:W90–W97
Lai M-C, Lee Y-HW, Tarn W-Y (2008) The DEAD-Box RNA helicase DDX3 associates with export messenger ribonucleoproteins as well asTip-associated protein and participates in translational control. Mol Biol Cell 19:3847–3858
Lee YH, Tan HT, Chung MCM (2010) Subcellular fractionation methods and strategies for proteomics. Proteomics 10:3935–3956
Lessley BA, Garner DL (1983) Isolation of motile spermatozoa by density gradient centrifugation in Percoll®. Gamete Res 7:49–61
Li HJ, Yu N, Zhang XY, Jin W, Li HZ (2010) Spermatozoal protein profiles in male infertility with asthenozoospermia. Chin Med J (engl) 123:2879–2882
Liu X, Teng Z, Wang Z, Zhu P, Song Z, Liu F (2022) Expressions of HSPA1L and HSPA9 are associated with poor sperm quality of low-motility spermatozoa in fertile men. Andrologia 54:e14321
Luconi M, Baldi E (2003) How do sperm swim? Molecular mechanisms underlying sperm motility. Cell Mol Biol (Noisy-le-Grand, France) 49:357–369
Luo W, Pant G, Bhavnasi YK, Blanchard SG Jr, Brouwer C (2017) Pathview Web: user friendly pathway visualization and data integration. Nucleic Acids Res 45:W501–W508
Macleod G, Varmuza S (2013) The application of proteomic approaches to the study of mammalian spermatogenesis and sperm function. Febs J 280:5635–5651
Manfrevola F, Ferraro B, Sellitto C, Rocco D, Fasano S, Pierantoni R, Chianese R (2021) CRISP2, CATSPER1 and PATE1 expression in human asthenozoospermic semen. Cells 10:1956
Martínez-Heredia J, de Mateo S, Vidal-Taboada JM, Ballesca JL, Oliva R (2008) Identification of proteomic differences in asthenozoospermic sperm samples. Hum Reprod 23:783–791
Martínez-Heredia J, Estanyol JM, Ballescà JL, Oliva R (2006) Proteomic identification of human sperm proteins. Proteomics 6:4356–4369
Mast FD, Ratushny AV, Aitchison JD (2014) Systems cell biology. J Cell Biol 206:695–706
Matsumura T, Endo T, Isotani A, Ogawa M, Ikawa M (2019) An azoospermic factor gene, Ddx3y and its paralog, Ddx3x are dispensable in germ cells for male fertility. J Reprod Dev 2018–2145
Mirzaei M, Abyadeh M, Turner AJ et al (2022) Fingolimod effects on the brain are mediated through biochemical modulation of bioenergetics, autophagy, and neuroinflammatory networks. Proteomics 22(19-20):e2100247. https://doi.org/10.1002/pmic.202100247
Mirzaei M, Gupta VB, Chick JM, Greco TM, Wu Y, Chitranshi N, Vander Wall R, Hone E, Deng L, Dheer Y (2017) Age-related neurodegenerative disease associated pathways identified in retinal and vitreous proteome from human glaucoma eyes. Sci Rep 7:1–16
Mobberley M (2010) Electron microscopy in the investigation of asthenozoospermia. Br J Biomed Sci 67:92–100
Müller-McNicoll M, Rossbach O, Hui J, Medenbach J (2019) Auto-regulatory feedback by RNA-binding proteins. J Mol Cell Biol 11:930–939
Organization WH (2010a) Department of reproductive health and research. WHO Laboratory Manual for the Examination and Processing of Human Semen 5:21–22
Boitrelle F, Shah R, Saleh R et al (2021) The sixth edition of the WHO manual for human semen analysis: A critical review and SWOT analysis. Life (Basel) 11(12):1368. Published 2021 Dec 9. https://doi.org/10.3390/life11121368
Ortega C, Verheyen G, Raick D, Camus M, Devroey P, Tournaye H (2011) Absolute asthenozoospermia and ICSI: what are the options? Hum Reprod Update 17:684–692
Padmanabhan PK, Zghidi-Abouzid O, Samant M, Dumas C, Aguiar BG, Estaquier J, Papadopoulou B (2016) DDX3 DEAD-box RNA helicase plays a central role in mitochondrial protein quality control in Leishmania. Cell Death Dis 7:e2406–e2406
Paulo JA, Gaun A, Kadiyala V, Ghoulidi A, Banks PA, Conwell DL, Steen H (2013) Subcellular fractionation enhances proteome coverage of pancreatic duct cells. Biochim Biophys Acta 1834:791–797
Rahimizadeh P, Topraggaleh TR, Nasr-Esfahani MH, Ziarati N, Mirshahvaladi S, Esmaeili V, Seifi S, Eftekhari-Yazdi P, Shahverdi A (2020) The alteration of PLCζ protein expression in unexplained infertile and asthenoteratozoospermic patients: a potential effect on sperm fertilization ability. Mol Reprod Dev 87:115–123
Ramalho-Santos J, Varum S, Amaral S, Mota PC, Sousa AP, Amaral A (2009) Mitochondrial functionality in reproduction: from gonads and gametes to embryos and embryonic stem cells. Hum Reprod Update 15:553–572
Raudvere U, Kolberg L, Kuzmin I, Arak T, Adler P, Peterson H, Vilo J (2019) g:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res 47:W191–W198
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK (2015) limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43:e47–e47
Roy A, Lin YN, Agno JE, DeMayo FJ, Matzuk MM (2007) Absence of tektin 4 causes asthenozoospermia and subfertility in male mice. Faseb J 21:1013–1025
Roy A, Lin YN, Agno JE, DeMayo FJ, Matzuk MM (2009) Tektin 3 is required for progressive sperm motility in mice. Mol Reprod Dev Inc Gamete Res 76:453–459
Salvolini E, Buldreghini E, Lucarini G, Vignini A, Lenzi A, Di Primio R, Balercia G (2013) Involvement of sperm plasma membrane and cytoskeletal proteins in human male infertility. Fertil Steril 99:697–704
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Sendler E, Johnson GD, Mao S, Goodrich RJ, Diamond MP, Hauser R, Krawetz SA (2013) Stability, delivery and functions of human sperm RNAs at fertilization. Nucleic Acids Res 41
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504
Shen S, Wang J, Liang J, He D (2013) Comparative proteomic study between human normal motility sperm and idiopathic asthenozoospermia. World J Urol 31:1395–1401
Shih J-W, Wang W-T, Tsai T-Y, Kuo C-Y, Li H-K, Wu Lee Y-H (2011) Critical roles of RNA helicase DDX3 and its interactions with eIF4E/PABP1 in stress granule assembly and stress response. Biochem J 441:119–129
Siva AB, Kameshwari DB, Singh V, Pavani K, Sundaram CS, Rangaraj N, Deenadayal M, Shivaji S (2010) Proteomics-based study on asthenozoospermia: differential expression of proteasome alpha complex. Mol Hum Reprod 16:452–462
Storey BT (2004) Mammalian sperm metabolism: oxygen and sugar, friend and foe. Int J Dev Biol 52:427–437
Tanaka H, Iguchi N, Toyama Y, Kitamura K, Takahashi T, Kaseda K, Maekawa M, Nishimune Y (2004) Mice deficient in the axonemal protein Tektin-t exhibit male infertility and immotile-cilium syndrome due to impaired inner arm dynein function. Mol Cell Biol 24:7958–7964
Taylor CF, Paton NW, Lilley KS, Binz P-A, Julian RK, Jones AR, Zhu W, Apweiler R, Aebersold R, Deutsch EW (2007) The minimum information about a proteomics experiment (MIAPE). Nat Biotechnol 25:887–893
Turner RM (2003) Tales from the tail: what do we really know about sperm motility? J Androl 24:790–803
Turner RM (2005) Moving to the beat: a review of mammalian sperm motility regulation. Reprod Fertil Dev 18:25–38
Wasinger VC, Zeng M, Yau Y (2013) Current status and advances in quantitative proteomic mass spectrometry. Int J Proteomics 2013
Wilhelm M, Schlegl J, Hahne H, Gholami AM, Lieberenz M, Savitski MM, Ziegler E, Butzmann L, Gessulat S, Marx H (2014) Mass-spectrometry-based draft of the human proteome. Nature 509:582–587
Wright P, Noirel J, Ow S-Y, Fazeli A (2012) A review of current proteomics technologies with a survey on their widespread use in reproductive biology investigations. Theriogenology 77(738–765):e752
Yates JR 3rd, Gilchrist A, Howell KE, Bergeron JJ (2005) Proteomics of organelles and large cellular structures. Nat Rev Mol Cell Biol 6:702–714
Zhao C, Huo R, Wang F-Q, Lin M, Zhou Z-M, Sha J-H (2007) Identification of several proteins involved in regulation of sperm motility by proteomic analysis. Fertil Steril 87:436–438
Zhu C, Yang Q, Xu J, Zhao W, Zhang Z, Xu D, Zhang Y, Zhao E, Zhao G (2019) Somatic mutation of DNAH genes implicated higher chemotherapy response rate in gastric adenocarcinoma patients. J Transl Med 17:109
Zuccarello D, Ferlin A, Garolla A, Pati MA, Moretti A, Cazzadore C, Francavilla S, Foresta C (2008) A possible association of a human tektin-t gene mutation (A229V) with isolated non-syndromic asthenozoospermia: case report. Hum Reprod 23:996–1001
Acknowledgements
We express our heartfelt appreciation to Morteza Abyadeh for his great help in writing this manuscript. We also thank Mehdi Alikhani for helping with preliminary data analysis and providing valuable contributions.
Funding
This work was supported by a research grant (91000398) from the Royan Institute.
Author information
Authors and Affiliations
Contributions
TRT and ASH conceived the idea and designed the study. SM performed mass spectrometry and bioinformatic analysis. TRT, SM, and PR carried out the experiments. The first draft was written by TRT and completed by MNB. All authors read and edited the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
This case–control study was approved by the Institutional Ethics Committee of Royan Institute, Tehran, Iran (IR.ACECR.ROYAN.REC.1391.398).
Consent to participate
The written informed consents were signed before semen collection by patients referring to Royan Institute for assisted reproduction treatments. All cases were informed about using their clinical and biological data for this study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mirshahvaladi, S., Topraggaleh, T.R., Bucak, M.N. et al. Quantitative proteomics of sperm tail in asthenozoospermic patients: exploring the molecular pathways affecting sperm motility. Cell Tissue Res 392, 793–810 (2023). https://doi.org/10.1007/s00441-023-03744-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-023-03744-y