Abstract
Ciliary neurotrophic factor (CNTF) is a pleiotropic cytokine that signals through a receptor complex containing a specific subunit, CNTF receptor α (CNTFRα). The two molecules are constitutively expressed in key structures for human placental growth and differentiation. The possible role of CNTF in enhancing cell proliferation and/or invasion during placental development and remodelling was investigated using HTR-8/SVneo and BeWo cells, taken respectively as cytotrophoblast and syncytiotrophoblast models. In both cell lines, treatment with human recombinant (hr) CNTF activated JAK2/STAT3 signalling and inhibited the ERK pathway. Interestingly, in HTR-8/SVneo cells, 50 ng hrCNTF induced significant downregulation of matrix metalloprotease (MMP)-1 and significant upregulation of MMP-9. Moreover, pharmacological inhibition of JAK2/STAT3 signalling by AG490 and curcumin resulted in MMP-9 downregulation; it activated the ERK signalling pathway and upregulated MMP-1 expression. Collectively, these data suggest a role for CNTF signalling in extravillous cytotrophoblast invasion through the modulation of specific MMPs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development and differentiation of the human placental villous tree are regulated by numerous growth factors, their receptors and other types of molecules, such as matrix metalloproteases (MMPs) (Chen and Khalil 2017), whose balanced action is critical for normal placental development and successful pregnancy. MMPs play an important role in the degradation and remodelling of the decidual extracellular matrix and are critical for trophoblast adhesion, invasion and proliferation (Espino et al. 2017). MMPs are a large family of zinc-dependent endopeptidases that include gelatinases (e.g. MMP-2, MMP-9), collagenases (e.g. MMP-1, MMP-8 and MMP-13), matrilysins (e.g. MMP-7, MMP-26), stromelysins (e.g. MMP-3, MMP-10) and type I and II transmembrane metalloproteinases (Nagase et al. 2006). In particular, altered MMP-1, -2, -3 and -9 regulation during placentation may lead to placental disease such as pregnancy loss (Balci and Ozdemir 2019), preterm delivery (Sundrani et al. 2012), preeclampsia (PE) (Sahay et al. 2018) and intra-uterine growth restriction (IUGR) (Swierczewski et al. 2012). A role for ciliary neurotrophic factor (CNTF) in MMP modulation has been demonstrated in murine neuroblastoma cell models, where CNTF downregulated MMP-2, thus potentially to affecting invasive behaviour (Sartor et al. 2002). CNTF is a 22-kDa cytokine encoded by the CNTF gene located on human chromosome 11 (Lam et al. 1991). It belongs to the interleukin (IL)-6 cytokine family along with cardiotrophin-1, IL-6, IL-11, IL-27, leukaemia inhibitory factor (LIF) and oncostatin-M (Heinrich et al. 2003). All the members of this family share glycoprotein 130 (gp130) as a signal-transducing receptor protein (Rose-John 2015). At the cellular level, the effects of CNTF are mediated via a receptor complex consisting of gp130, LIF receptor ß (LIFRß) and CNTF receptor α (CNTFRα) (Wagener et al. 2014). CNTFRα is widely expressed in neuronal cells (Ding et al. 2013), hepatocytes (Wang and Fuller 1995), adipocytes (Perugini et al. 2019), muscle (Steinberg et al. 2009), retinal (Ghasemi et al. 2018) and prostatic cells (Fantone et al. 2020b) and pancreatic β-cells (Rezende et al. 2009), where it plays key and complex roles in cell proliferation and differentiation and helps cells survive pathological stimuli (Ding et al. 2013).
The role of the IL-6 cytokine family in pregnancy has been extensively investigated (Ge et al. 2018; Horita et al. 2007; Markert et al. 2011). Interestingly, IL-6 and/or IL-6R dysregulation has been associated with impaired embryo implantation and abortion (Markert et al. 2011). In addition, IL-6 plays a pivotal role in regulating the proliferation and invasiveness of cytotrophoblast cells by increasing MMP-9 and MMP-2 activity (Meisser et al. 1999).
The IL-6 family members, particularly CNTF, can trigger different intracellular signalling events that depend on cell type, including activation of extracellular signal-regulated kinases (ERK) (Trimarchi et al. 2009), of the phosphatidylinositol 3 kinase (PI3K)-AKT pathway (Hiatt et al. 2014) and of the Janus kinase (JAK)2/signal transducer and activator of transcription (STAT)-3 signalling pathway (Rezende et al. 2009). Notably, CNTF can simultaneously activate multiple signalling pathways in the same cell type (Steinberg et al. 2009; Trimarchi et al. 2009).
Very few studies have explored a role for CNTF in pregnancy. A study of the circulating levels of CNTF in non-pregnant and pregnant women and in women with pregnancies complicated by PE has found that CNTF levels were lower in pregnant compared with non-pregnant women, that maternal CNTF blood levels tended to decline with the progression of normal pregnancy and that they were lower in pregnancies complicated by PE than in normal pregnancies (Akahori et al. 2010). In contrast, Bienertova-Vasku and colleagues showed no difference in maternal CNTF blood levels between normal and PE pregnancies at term, but described higher CNTF levels in umbilical cord foetal blood of PE compared with normal pregnancies (Bienertova-Vasku et al. 2013). Unfortunately, in the latter study, the samples were not matched for gestational age, thus preventing all comparison. We hypothesize that the lower CNTF levels found in maternal plasma during gestation and the altered CNTF levels described in pregnancies complicated by PE can be related to placental development during gestation. This study was devised to assess the presence of CNTF in maternal blood and understand its action on placental development. First we identified the placental structures expressing CNTF and CNTFRα in first- and third-trimester human placentas and then investigated CNTF involvement in placental development by testing its possible modulation of MMP-1, MMP-2 and MMP-9 (Chen and Khalil 2017).
Materials and methods
Tissue collection
We examined 24 placentas, 12 from pregnant women undergoing voluntary pregnancy termination at 9–12 weeks of gestation (Obstetrics and Gynaecology of San Severino Hospital, Macerata, Italy) and 12 from third-trimester pregnancies that were terminated by caesarean section (Department of Woman and Child Health, A. Gemelli Hospital, Università Cattolica del Sacro Cuore Rome, Italy and Obstetrics and Gynaecology, Department of Clinical Sciences, Polytechnic University of Marche, Ancona, Italy). In parallel, we also analysed first-trimester placentas from clinically normal pregnancies interrupted by aspiration curettage for psychosocial or medical reasons that were unlikely to affect placental structure or function. All women gave their written informed consent to the collection of placental specimens. The collection procedures were in accordance with the Helsinki Declaration of 1975, as revised in 2013. The permission of the Human Investigation Committee of the Marche Region (Italy) was obtained (protocol no. 2019.172; study ID 980; CERM no. 172).
Immediately after delivery and gross examination, three zones were identified in each placenta: a central zone near the umbilical cord insertion, a peripheral zone, i.e. the most distal from the umbilical cord, and an intermediate zone. Two tissue samples were collected from each zone. The samples for immunohistochemistry were fixed in 4% buffered formalin at 4 °C for 12 h and routinely processed for paraffin embedding at 56° C, whereas the samples for biochemical and molecular analysis were frozen in liquid nitrogen as described previously (Fantone et al. 2020a; Tossetta et al. 2019).
Immunohistochemical analysis of first- and third-trimester placentas
Immunohistochemical staining was performed as described previously (Altobelli et al. 2017; Avellini et al. 2017). Briefly, after dewaxing, paraffin sections were rinsed in phosphate buffered saline (PBS) and incubated with 3% hydrogen peroxide for 40 min to block endogenous peroxidase. They were then subjected to high-temperature pretreatment in 10 mM citrate buffer, pH 6.0, for 5 min (CNTF) or in 100 ng/ml proteinase K (Sigma‐Aldrich, St. Louis, MO, USA) for 5 min at 37 °C (CNTFRα). Sections were subsequently rinsed with PBS and incubated with normal horse serum (Vector Laboratories, Burlingame, CA, USA) diluted 1:75 in PBS for 1 h at room temperature (RT). This was followed by incubation with the primary antibodies (Table 1) diluted in PBS and then overnight incubation at 4 °C. After a thorough rinse in PBS, sections were incubated in a 1:200 biotinylated secondary antibody (Vector Laboratories) solution for 30 min at RT. Histochemical reactions were performed using Vectastain ABC Kit (Vector Laboratories) for 1 h at RT with 3′,3′‐ diaminobenzidine hydrochloride (Sigma‐Aldrich) as the chromogen. Sections were counterstained with Mayer’s haematoxylin, dehydrated and mounted in Eukitt (Kindler GmbH and Co., Freiburg, Germany). Negative controls were performed by replacing the primary or the secondary antibody with an isotype antibody: monoclonal rabbit IgG (ab172730; Abcam, Cambridge, UK) for CNTF and monoclonal mouse IgG2a (ab18415; Abcam) for CNTFRα.
Culture of HTR-8/SVneo and BeWo cell lines
To test the involvement of CNTF in proliferation and invasion processes, we used the cytotrophoblast cell line HTR-8/SVneo (kindly provided by C. H. Graham, Queen’s University, Kingston, ON, Canada) and the choriocarcinoma derived BeWo human placental cell line (kindly provided by S. Alberti, Laboratory of Cancer Pathology, CeSI-MeT, University ‘G. d’Annunzio’, Chieti, Italy). BeWo cells express an epithelial proteome characteristic of villous trophoblasts, whereas HTR-8/SVneo cells exhibit a phenotype characteristic of extravillous trophoblasts (Szklanna et al. 2017). Notably, BeWo cells also show a spontaneous ability to form syncytial units mimicking the syncytiotrophoblast, whereas a marked self-renewal ability makes HTR-8/SVneo cells a good model of cytotrophoblast cells (Weber et al. 2013). Although freshly isolated cytotrophoblast can differentiate into multinucleated syncytiotrophoblast, their inability to proliferate in vitro hampers experiments and prevents their use (Chen et al. 2013; King et al. 2000; Kohli et al. 2016; Mannelli et al. 2015).
HTR-8/SVneo cells (Graham et al. 1993) were cultured in RPMI 1640 medium (Life Technologies, Monza, Italy), whereas BeWo cells were cultured in DMEM/F12 medium. Both media were supplemented with 10% foetal bovine serum (FBS) and 100 U/ml penicillin and streptomycin (all from Gibco, Thermo Fisher Scientific, Waltham, MA, USA). All cultures were incubated at 37 °C with 95% humidity and 5% CO2. The culture media were changed twice a week.
Immunofluorescence of HTR-8/SVneo and BeWo cell lines
HTR-8/SVneo and BeWo cells were washed in Dulbecco’s PBS (Life Technologies, Monza, Italy), fixed in 4% paraformaldehyde in PBS for 10 min at 4 °C and permeabilized in 0.1 M PBS added with 0.1% Triton X-100 (Sigma, Milano, Italy) for 5 min. After washing in PBS at RT, cells were blocked with 10% normal donkey serum (Jackson ImmunoResearch, Cambridgeshire, UK) in 0.1 M PBS and incubated overnight at 4 °C with anti-human CNTF and CNTFRα antibodies (Table 1). Cells were then washed 3 times in PBS and incubated with Alexa Fluor 488-conjugated donkey anti-rabbit (for CNTF) and Alexa Fluor 555-conjugated donkey anti-mouse (for CNTFRα) IgG secondary antibody (both from Thermo Fisher Scientific) for 30 min at RT. The TOTO3 probe was used for nuclear staining. Finally, the slides were cover-slipped with propyl gallate and evaluated with an Eclipse E600 fluorescence microscope (Nikon, Düsseldorf, Germany).
Treatment of HTR-8/SVneo and BeWo cell lines with rhCNTF
Both placental cell lines express CNTFRα. After seeding at confluence (1.5 104/cm2), they were incubated with 20, 50 or 100 ng/ml recombinant human (rh) CNTF (cat. #257-NT-010, R&D Systems, Minneapolis, MN, USA) for 15 min, to investigate the possible signalling pathways triggered by CNTF. We focused on 3 possible pathways where the IL-6 cytokine family is commonly involved, the JAK2/STAT3, PI3K/AKT and MAPK/ERK pathways.
The two cell lines were also treated with two pSTAT3 inhibitors, AG490 and curcumin (Merck, Darmstadt, Germany), to assess the specificity of CNTF action. To do so, HTR-8/SVneo cells were incubated with 100 µM AG490 or 15 µM curcumin with/without 50 ng/ml rhCNTF for 24 h in suitable media. The doses were chosen after evaluation of pSTAT3 inhibition in a dose–response curve.
Viable counts were routinely performed using the trypan blue dye exclusion test. All experiments were conducted in triplicate and were repeated at least 3 times.
Protein extracts and Western blotting of human placental samples and cell lines
After thawing, the placental samples were washed with 0.1 M PBS, pH 7.4. Each sample (300 mg) was homogenized in an Ultra Turrax T8 homogenizer (IKA-WERKE, Lille, France) using a lysis buffer consisting of 0.1 M PBS, 0.1% (w/v) SDS, 1% (w/w) NONIDET‐P40, 1 mM (w/v) Na orthovanadate, 1 mM (w/w) phenyl methane sulfonyl fluoride (PMSF), 12 mM (w/v) Na deoxycholate and 1.7 μg/ml aprotinin, pH 7.5. The placenta samples and the treated and untreated HTR-8/SVneo and BeWo cells were lysed in lysis buffer, centrifuged at 10,000 g for 20 min at 4 °C, aliquoted and stored at − 80 °C until use. Protein concentrations were determined by a Bradford protein assay (Bio‐Rad Laboratories, Milano, Italy). All protein samples were fractionated on 10% SDS–polyacrylamide gels (SDS-PAGE) as described previously (Laemmli 1970), electrophoretically transferred (Trans-Blot® Turbo™ Transfer System; Bio-Rad Laboratories) to nitrocellulose membranes, and subjected to Western blot analysis. Non-specific protein binding was blocked with 5% non‐fat‐dried milk (Bio‐Rad Laboratories) in Tris‐buffered saline TBS/0.05% Tween 20 (TBS‐T) for 1 h. Blots were incubated with the primary antibodies listed in Table 1. After washing, blots were incubated with appropriate secondary antibodies conjugated to horseradish peroxidase (Amersham Italia s.r.l., Milano, Italy) diluted 1:5000 in TBS-T. Bound antibodies were detected with Clarity Western ECL Substrate (Bio‐Rad Laboratories). Images were acquired with Chemidoc (Bio‐Rad Laboratories). Band densitometric analysis was performed using ImageJ software (National Institutes of Health; https://imagej.nih.gov/ij/download.html).
Analysis of HTR-8/SVneo cell cycle after hrCNTF treatment
To examine the role of rhCNTF in cell proliferation, HTR-8/SVneo cells were treated with 50 ng/ml hrCNTF for 24 h, suspended at 1 × 106 cells/ml in 1 ml PBS and centrifuged at 200 g for 5 min at RT. PBS was then removed and cells were fixed by adding 4.5 ml 70% cold ethanol (− 20 °C) overnight at 4 °C. After fixation, cells were centrifuged at 400 g for 5 min; pellets were washed 3 times with 5 ml PBS and centrifuged at 400 g for 5 min. After removing the supernatant, cells were resuspended in 1 ml propidium iodide solution (Miltenyi Biotec, Bergisch Gladbach, Germany) and incubated for 30 min at RT in the dark. Approximately 20,000 cells/sample were analysed using FacsCanto II (DB Biosciences, San Jose, CA, USA) with excitation at 488 nm and emission at 617 nm. The percentage of cells in each phase of the cell cycle was determined using FacsDiva Software (DB Biosciences).
Wound healing assay
To investigate the role of CNTF in cell motility, HTR-8/SVneo cells grown to confluence were scratch-wounded with a sterile plastic micropipette tip. Cells were rinsed 3 times with warm medium to remove any scraped off cells in the wound and then treated/not treated with 50 ng/ml rhCNTF for 24 h. Digital images were taken at 0, 4 and 24 h. The area of the wound not occupied by cells was measured using ImageJ software. The experiment was repeated at least 3 times.
Transwell invasion assay
Matrigel invasion assays were performed using 24-well transwell inserts with 8.0 μm pores (cat. no. 353097) coated with 100 µl of 250 µg/ml LDEV-free Matrigel (cat. no. 356234; all from Corning, NY, USA) diluted in serum-free RPMI 1640 medium according to the manufacturer’s instructions. The effects of CNTF on invasiveness were assessed by pretreating HTR-8/SVneo cells with 50 ng/ml CNTF for 24 h before seeding in the transwell chambers. In the upper chambers, 5 × 104 cells were seeded on top of the Matrigel and supplemented with 200 µl serum-free RPMI 1640, with/without 50 ng/ml CNTF. The lower chambers contained 750 µl of the medium with a chemoattractant (10% FBS). After 24 h, non-invading cells and Matrigel were gently removed from the apical side of the membrane by scrubbing with a cotton swab, whereas the cells remaining on the lower surface were fixed with 100% methanol for 20 min and stained with 0.5% crystal violet for 15 min. Four non-overlapping fields of view per sample were captured at 10 × magnification and cells were counted using ImageJ software. Data were expressed as mean cell number in each of the four fields. The experiments were repeated 3 times in triplicate.
Statistical analysis
Data are reported as mean ± standard deviation (SD). Statistical significance was analysed using Student’s t-test (p < 0.05). Statistical analyses were performed with GraphPad Prism (ver. 8) software.
Results
CNTF and CNTFRα were expressed in normal human placenta and in human placenta cell models (HTR-8/SVneo and BeWo)
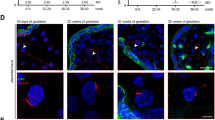
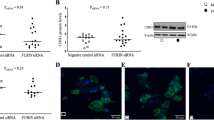
In first-trimester placentas, CNTF (Fig. 1a, b) and CNTFRα (Fig. 1e, f) immunohistochemistry showed that both molecules were expressed in the cytotrophoblast layer (Fig. 1a, f: arrows), in the foetal vessels (see, for example, CNTFRα staining in Fig. 1e: V) and in the extravillous cytotrophoblast of cell columns and islands (see, for example, CNTF staining in Fig. 1b, where a column is indicated by asterisks). The syncytiotrophoblast of first-trimester placentas was negative for both CNTF and CNTFRα. In striking contrast, in third-trimester placentas, CNTF (Fig. 1c, d) and CNTFRα (Fig. 1g, h) were detected in the syncytiotrophoblast layer (Fig. 1d, h: arrowheads), whereas cytotrophoblast and endothelial cells were negative for both molecules. CNTF (Fig. 2a) and CNTFRα (Fig. 2b) were identified in the Western blots of first- and third-trimester placentas, but showed no significant differences in expression (a’, b’). By immunofluorescence analysis, HTR-8/SVneo and BeWo cells (respectively in vitro villous cytotrophoblast and syncytiotrophoblast models) were positive for both CNTF (Fig. 3 green staining) and CNTFRα (Fig. 3 red staining). In particular, CNTF was expressed in the nuclei and the cytoplasm of both cell lines (HTR-8/SVneo cells, Fig. 3a–c; BeWo cells, Fig. 3g–i), whereas CNTFRα staining was limited to the cytoplasm, especially the perinuclear region (HTR-8/SVneo cells, Fig. 3d–f; BeWo cells, Fig. 3k–l).
Immunohistochemistry of CNTF (a–d) and CNTFRa (e–h) in normal first-trimester (a, b, e, f) and third-trimester (c, d, g, h) placentas. a In first-trimester placental villi, CNTF is expressed in villous cytotrophoblast cells (arrows) whereas the syncytiotrophoblast is negative. b The extravillous cytotrophoblast cells are CNTF-positive, showing intense staining in the proximal portion of cell columns, near the villus (*). In third-trimester placental villi, only the syncytiotrophoblast (arrowheads) shows immunostaining (c, d). In (d), a terminal villus shows that the syncytiotrophoblast is CNTF-positive. In first-trimester placental villi, CNTFRa (e, f) is expressed in villous cytotrophoblast cells (arrows) whereas the syncytiotrophoblast is negative. Foetal vessels are CNTFRa-positive (e: V). g, h In third-trimester placental villi, only the syncytiotrophoblast (arrowheads) shows CNTFRa immunostaining. i, j Negative controls. b Bar = 50 mm; c, e, g, i, j bars = 100 mm; a, f, h bars = 30 mm; d bar = 25 mm
Western blots showing CNTF (a) and CNTFRa (b) protein expression in first- and third-trimester normal placentas. Bands (a, b) were analysed densitometrically (a’, b’). Results are expressed in arbitrary units (A.U.) and shown as histogram bars (a’, b’). CNTF and CNTFRa quantities were normalized using β‐actin expression. 5 ng of human hrCNTF was used as positive control (not shown). There were no significant differences in CNTF and CNTFRa protein expression between the first (I Trim) and third (III Trim) trimester (n = 12 for I Trim; n = 12 for III Trim)
CNTF and CNTFRa immunofluorescence in HTR-8/SVneo (a–f) and BeWo (g–l) cells. CNTF immunostaining (green) is localized in the cytoplasm and nucleoplasm of both HTR-8/SVneo (b–c) and BeWo (h–i) cells, whereas the nucleoli are CNTF-negative. CNTFRα immunostaining (red) is localized in the cytoplasm and is especially intense in the perinuclear region of both HTR-8/SVneo (e–f) and BeWo (k–l) cells. The nuclei are stained blue. a–c, g–l Bars = 100 mm; d–f bars = 50 mm. Negative control: c’, f’, i’, l’ bars = 100 mm (HTR-8/SVneo) and 50 mm (BeWo)
Effect of CNTF on MMPs modulation
rhCNTF activated the JAK2/STAT3 pathway in a dose-dependent manner in both HTR-8/SVneo (Fig. 4a–c) and BeWo (Fig. 4d–f) cell cultures; in particular, a significantly increased pSTAT3 expression was already detected after treatment with 20 ng/ml rhCNTF (Fig. 4a’, d’). Interestingly, the CNTF treatments did not induce modulation of AKT signalling (Fig. 4b’, e’), whereas they did inhibit the MAPK/ERK pathway, with a statistically significant effect at 50 and 100 ng/ml rhCNTF (Fig. 4c’, f’). The 50 ng/ml concentration, which can activate the JAK2/STAT3 pathway and inhibit MAPK/ERK signalling, induced significant downregulation of MMP-1 (Fig. 5a, a’) and significant upregulation of MMP-9 (Fig. 5c, c’) in HTR-8/SVneo cells, but exerted no significant effect on BeWo cells (Fig. 5e, e’). Since these data suggest that MMP-1 and MMP-9 expression may be under the control of MAPK/ERK and JAK2/STAT3 signalling, respectively, we investigated the role of the two pathways in modulating MMP-1 and MMP-9 protein expression using AG490 as a JAK2 inhibitor and curcumin as a STAT3 inhibitor (Rezende et al. 2009). As expected, AG490 (Fig. 6a–d) and curcumin (Fig. 6e–h) significantly inhibited pSTAT3 expression (Fig. 6a’, e’) and increased ERK1/2 activation (Fig. 6b’, f’), resulting in MMP-1 upregulation (Fig. 6e’, g’) and MMP-9 downregulation (Fig. 6d’, h’). rhCNTF treatment induced opposite effects, suggesting that the reduced MMP-1 expression was due to suppression of MAPK/ERK signalling, whereas the increased MMP-9 expression was related to JAK2/STAT3 activation.
In HTR-8/SVneo and BeWo cells, CNTF induced pSTAT3 upregulation and pERK downregulation. Left, Western blots (a–f); right, histograms (a’–f’) showing band quantification. a, a’, b, b’, c, c’ HTR-8/SVneo cells and d, d’, e, e’, f, f’ BeWo cells after treatment with 20, 50 or 100 ng/ml rhCNTF. a’, d’ significant increase in pSTAT3 protein levels; b’, e’ unchanged pAKT protein levels; c’, f’ significant reduction in pERK protein levels. Results are expressed in arbitrary units (A.U.) and reported as histogram bars. pSTAT3, pAKT and pERK1/2 quantities were normalized using total STAT3, AKT and ERK1/2, respectively. Data are mean ± SD (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
In HTR-8/SVneo cells, CNTF treatment induced MMP-1 downregulation and MMP-9 upregulation. Left, Western blots (a–e); right, histograms (a’–e’) showing band quantification. a, a’, b, b’, c, c’ HTR-8/SVneo cells and d, d’, e, e’ BeWo cells after treatment with 50 ng/ml rhCNTF. a’ Significant MMP-1 downregulation; b’ lack of significant differences in MMP-2; c’ significant MMP-9 upregulation; d’ lack of significant differences in MMP-2 protein levels; e’ unchanged MMP-9 protein expression. MMP-1, MMP-2 and MMP-9 quantities were normalized using β‐actin. Results are expressed in arbitrary units (A.U.) and reported as histogram bars. Data are mean ± SD (n = 3). *p < 0.05
MMP-1 expression is mediated by the MAPK/ERK pathway whereas MMP-9 expression is mediated by the JAK2/STAT3 pathway. Left, Western blots (a–h); right, histograms (a’–h’) showing band quantification. Use of AG490 (a–d) and curcumin (CURC) (e–h) to inhibit the pSTAT3 pathway. (a’, e’) pSTAT3 inhibition was related to MMP-9 downregulation (d’, h’) and pERK (b’, f’) and MMP-1 (c’, g’) upregulation. pSTAT3 and pERK quantities were normalized using total STAT3 and ERK1/2, respectively. MMP-1 and MMP-9 quantities were normalized using β-actin. Results are expressed in arbitrary units (A.U.) and reported as histogram bars. Data are mean ± SD (n = 3). *p < 0.05, **p < 0.01
CNTF did not modulate HTR-8/SVneo cell proliferation
The cell cycle phases did not exhibit significant changes between cells treated with 50 ng/ml hrCNTF for 24 h and untreated cells (Fig. 7a). This finding was further confirmed by the analysis of PCNA expression in the Western blots, which was not significantly different in treated and untreated cells (Fig. 7b).
The HTR-8/SVneo cell cycle was not changed by CNTF treatment. Graphs representing untreated (a) and CNTF-treated (a’) HTR-8/SVneo cells. The area under the green curve represents the G0/G1 phases, the red curve the S phase and the blue curve the G2/M phases of the cell cycle. a’’ Histograms represent the percentage of the cells in each cell cycle phase and show that there were no significant differences in the cell cycle phases between treated and untreated cells. b PCNA Western blots of HTR-8/SVneo cells, untreated and CNTF-treated. Right, b’ histograms showing band quantification. There were no significant differences between untreated and CNTF-treated cells. Bands were normalized using β‐actin. Results are expressed in arbitrary units (A.U.) and reported as histogram bars. Data are mean ± SD (n = 3)
CNTF did not affect the motility and invasion of HTR-8/SVneo cells
To assess the effect of CNTF on cell migration, HTR-8/SVneo cells were treated with 50 ng/ml hrCNTF for 0, 4, 8 or 24 h (Fig. 8). The lack of significant differences in cell migration (Fig. 8e) suggested that CNTF is not involved in modulating villous cytotrophoblast migration. HTR-8/SVneo cells were also pretreated with 50 ng/ml hrCNTF for 24 h, to establish whether CNTF affects invasion properties. However, there was no significant difference in invasion ability between treated and untreated cells (Fig. 9). These data suggest that MMP-1 and MMP-9 modulation by CNTF did not alter cell motility and invasion in our in vitro model.
Wound closure experiments. CNTF did not affect HTR-8/SVneo cell mobility. a–d, a’–d’ Representative photographs show the effect of CNTF treatment on cell migration at different time points (0, 4, 8, 24 h). e The graph, representing the cell-free area, shows that CNTF treatment did not affect wound closure. In treated and untreated cells, wound closure was completed in 24 h. Results are expressed in arbitrary units (A.U.) and reported as histogram bars. Bars = 100 mm. Data are mean ± SD (n = 3)
Transwell invasion assays. CNTF did not affect HTR-8/SVneo cell invasion. Representative images showing the effect of CNTF (a’) on HTR-8/SVneo cell invasion (a, a’). b The graph shows that there were no differences in invasion between treated and untreated HTR-8/SVneo cells. Invading cells are expressed as mean ± SD (n = 3) of invading cells/field and reported as histogram bars. Bars = 100 µm
Discussion
The placenta is a vital organ with key roles in foetal development. In the first stages of gestation, the cytotrophoblast differentiates into extravillous and villous. The villous cytotrophoblast gives rise to the syncytiotrophoblast (Huppertz 2008). In differentiated placental villi at the end of gestation, the trophoblast surface consists of a continuous outer syncytiotrophoblast layer beneath which lies a discontinuous layer of villous cytotrophoblast cells. The villous cytotrophoblast is the proliferating stem cell that can differentiate into the syncytiotrophoblast, forming the maternal-foetal transport barrier. In contrast, extravillous trophoblast cells lie outside the villi and form cell columns and islands, which are the result of different stages of proliferation and differentiation and whose main function is to anchor the placenta to the uterine wall (Moser et al. 2018). Thus, the villous and extravillous cytotrophoblast is a key structure for the development of the placental villous tree and play a central role in growth and invasion processes during gestation (Ferretti et al. 2007; Muhlhauser et al. 1995), whereas the syncytiotrophoblast is responsible for foetal-maternal exchanges (Huppertz 2008). In this study, we found that in the first trimester of gestation CNTF and CNTFRα are expressed in both villous and extravillous cytotrophoblast cells, whereas in the third trimester their expression is confined to the syncytiotrophoblast. We propose that such consistent expression in structures vital for placental growth makes CNTF a constitutive protein with a potential role in the maintenance of placental morphology. A paracrine role for this cytokine linked to placental tissue remodelling during gestation cannot be excluded. In particular, the presence of CNTFRα in the foetal vessels suggests a possible role of CNTF in vascular remodelling as demonstrated in other tissues including the retina, where CNTF has been shown to exert an anti-angiogenic effect on endothelial cell sprouting through activation of the JAK/STAT3/SOCS3 signalling (Bucher et al. 2016).
In addition, it is also conceivable that in some conditions, CNTF may be secreted into the maternal circulation, thus acting as a hormone, as described previously (Akahori et al. 2010; Bienertova-Vasku et al. 2013).
It has clearly been documented that CNTF can modulate MMPs in human tissues (Chen et al. 2016) and that the invasive capacity of the trophoblast is strongly associated with its ability to produce and secrete MMPs (Fisher et al. 1989; Librach et al. 1991; Vegh et al. 1999). Here, we suggest that the presence of CNTFRα in the villous and extravillous cytotrophoblast may be related to the modulation of MMP-1 and MMP-9 through the action of CNTF. Interestingly, CNTF appears to regulate their expression through two different pathways. Our findings provide the first evidence of endogenous CNTF production, whose function seem to be the maintenance of placental tissue homeostasis. Notably, we demonstrated that MMP-1 downregulation was mediated by inhibition of MAPK/ERK signalling, whereas MMP-9 upregulation was mediated by activation of the JAK2/STAT3 pathway, in line with a recent report showing MMP-9 modulation by JAK2/STAT3 signalling in HTR-8/SVneo cells (Zong et al. 2016). We also demonstrated that CNTF did not affect cell proliferation, mobility and invasion, but only the balance of MMPs secretion.
So, MMPs effect was not detected in mobility and invasion processes probably due to the compensatory action of MMP-9 and MMP-1.
Collagen is a major substrate for MMPs and type I, III, IV and V collagens are expressed in human placenta (Oefner et al. 2015; Sati et al. 2008). In particular, type IV is chiefly expressed in the basal membrane of the placental villus, under the villous cytotrophoblast and around foetal vessels, whereas types I and III are localized in the interstitial stroma of foetal villi and in the extravillous trophoblast (Oefner et al. 2015; Sati et al. 2008). MMP-1 is an interstitial collagenase that degrades various interstitial collagens, including types I and III, thus playing a role in extravillous trophoblast invasion of the uterine wall. In contrast, MMP-9 is a type IV collagenase that principally degrades collagen type IV, in this respect it being similar to MMP-2 (Klein and Bischoff 2011). Notably, complete inhibition of in vitro trophoblast invasion by an MMP-9 antibody has suggested a key role for it in cell invasion processes (Librach et al. 1991). The proliferative ability of the villous cytotrophoblast and the extravillous cytotrophoblast are ensured by closeness to the basal membrane, where they form a compact cluster of cells. When villous cytotrophoblast cells leave the basal membrane, they lose their polarity and acquire an invasive phenotype that allows interaction with the surrounding extracellular matrix molecules, such as MMPs (Benirschke et al. 2006). Altogether, these data suggest that CNTF is involved in controlling the first phases of villous and extravillous cytotrophoblast migration by promoting collagen IV degradation by MMP-9 and by inhibiting cell mobility via MMP-1 downregulation. It is conceivable that MMP-9 upregulation by CNTF induces collagen type IV degradation which would be followed by a breach in the basal membrane and the switch of trophoblast cells to the invasive phenotype. The hypothesis is consistent with studies demonstrating that MMP-9 downregulation in the trophoblast plays an important role in PE onset, since a shallow extravillous trophoblast invasion hampers the transformation of uterine spiral arteries in the placental bed (Chen and Khalil 2017). In conclusion, we demonstrate that CNTF should be considered a constitutive placental cytokine acting in structures which are essential for human placental development and function, and that it plays a central role in modulating MMP-1 and MMP-9 expression by two different signalling pathways.
Change history
22 April 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00441-023-03771-9
References
Akahori Y, Takamoto N, Masumoto A, Inoue S, Nakatsukasa H, Masuyama H, Hiramatsu Y (2010) Circulating levels of ciliary neurotrophic factor in normal pregnancy and preeclampsia. Acta Med Okayama 64:129–136
Altobelli E, Latella G, Morroni M, Licini C, Tossetta G, Mazzucchelli R, Profeta VF, Coletti G, Leocata P, Castellucci M, Guerrieri M, Petrocelli R, De Berardis B, De Padova M, Di Leonardo G, Paladini A, Mignosi F, Quaglione G, Fagnano R, Marzioni D (2017) Low HtrA1 expression in patients with longstanding ulcerative colitis and colorectal cancer. Oncol Rep 38:418–426
Avellini C, Licini C, Lazzarini R, Gesuita R, Guerra E, Tossetta G, Castellucci C, Giannubilo SR, Procopio A, Alberti S, Mazzucchelli R, Olivieri F, Marzioni D (2017) The trophoblast cell surface antigen 2 and miR-125b axis in urothelial bladder cancer. Oncotarget 8:58642–58653
Balci M, Ozdemir G (2019) Differential expression of EGFR-1, MMP-3, and MMP-9 in spontaneous abortions, induced abortions, and tubal pregnancies. Turk Patoloji Derg 35:1–8
Benirschke K, Kaufmann P, Baergen RN (2006) Pathology of the human placenta. 207–209
Bienertova-Vasku J, Bienert P, Zlamal F, Splichal Z, Tomandl J, Tomandlova M, Hodicka Z, Ventruba P, Vasku A (2013) Brain-derived neurotrophic factor and ciliary neurotrophic factor in maternal plasma and umbilical cord blood from pre-eclamptic and physiological pregnancies. J Obstet Gynaecol 33:359–363
Bucher F, Walz JM, Buhler A, Aguilar E, Lange C, Diaz-Aguilar S, Martin G, Schlunck G, Agostini H, Friedlander M, Stahl A (2016) CNTF attenuates vasoproliferative changes through upregulation of SOCS3 in a mouse-model of oxygen-induced retinopathy. Invest Ophthalmol vis Sci 57:4017–4026
Chen J, Chen P, Backman LJ, Zhou Q, Danielson P (2016) Ciliary neurotrophic factor promotes the migration of corneal epithelial stem/progenitor cells by up-regulation of MMPs through the phosphorylation of Akt. Sci Rep 6:25870
Chen J, Khalil RA (2017) Matrix metalloproteinases in normal pregnancy and preeclampsia. Prog Mol Biol Transl Sci 148:87–165
Chen Y, Wang K, Leach R (2013) 5-Aza-dC treatment induces mesenchymal-to-epithelial transition in 1st trimester trophoblast cell line HTR8/SVneo. Biochem Biophys Res Commun 432:116–122
Ding J, He Z, Ruan J, Ma Z, Liu Y, Gong C, Iqbal K, Sun S, Chen H (2013) Role of ciliary neurotrophic factor in the proliferation and differentiation of neural stem cells. J Alzheimers Dis 37:587–592
Espino YSS, Flores-Pliego A, Espejel-Nunez A, Medina-Bastidas D, Vadillo-Ortega F, Zaga-Clavellina V, Estrada-Gutierrez G (2017) New insights into the role of matrix metalloproteinases in preeclampsia. Int J Mol Sci 18
Fantone S, Mazzucchelli R, Giannubilo SR, Ciavattini A, Marzioni D, Tossetta G (2020a) AT-rich interactive domain 1A protein expression in normal and pathological pregnancies complicated by preeclampsia. Histochem Cell Biol 154:339–346
Fantone S, Tossetta G, Montironi R, Senzacqua M, Marzioni D, Mazzucchelli R (2020b) Ciliary neurotrophic factor (CNTF) and its receptor (CNTFRalpha) signal through MAPK/ERK pathway in human prostate tissues: a morphological and biomolecular study. Eur J Histochem 64
Ferretti C, Bruni L, Dangles-Marie V, Pecking AP, Bellet D (2007) Molecular circuits shared by placental and cancer cells, and their implications in the proliferative, invasive and migratory capacities of trophoblasts. Hum Reprod Update 13:121–141
Fisher SJ, Cui TY, Zhang L, Hartman L, Grahl K, Zhang GY, Tarpey J, Damsky CH (1989) Adhesive and degradative properties of human placental cytotrophoblast cells in vitro. J Cell Biol 109:891–902
Ge H, Yin N, Han TL, Huang D, Chen X, Xu P, He C, Tong C, Qi H (2018) Interleukin-27 inhibits trophoblast cell invasion and migration by affecting the epithelial-mesenchymal transition in preeclampsia. Reprod Sci 1933719118799206
Ghasemi M, Alizadeh E, Saei Arezoumand K, Fallahi Motlagh B, Zarghami N (2018) Ciliary neurotrophic factor (CNTF) delivery to retina: an overview of current research advancements. Artif Cells Nanomed Biotechnol 46:1694–1707
Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK (1993) Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res 206:204–211
Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F (2003) Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J 374:1–20
Hiatt K, Lewis D, Shew M, Bijangi-Vishehsaraei K, Halum S (2014) Ciliary neurotrophic factor (CNTF) promotes skeletal muscle progenitor cell (MPC) viability via the phosphatidylinositol 3-kinase-Akt pathway. J Tissue Eng Regen Med 8:963–968
Horita H, Kuroda E, Hachisuga T, Kashimura M, Yamashita U (2007) Induction of prostaglandin E2 production by leukemia inhibitory factor promotes migration of first trimester extravillous trophoblast cell line, HTR-8/SVneo. Hum Reprod 22:1801–1809
Huppertz B (2008) The anatomy of the normal placenta. J Clin Pathol 61:1296–1302
King A, Thomas L, Bischof P (2000) Cell culture models of trophoblast II: trophoblast cell lines--a workshop report. Placenta 21 Suppl A:S113–119
Klein T, Bischoff R (2011) Physiology and pathophysiology of matrix metalloproteases. Amino Acids 41:271–290
Kohli S, Ranjan S, Hoffmann J, Kashif M, Daniel EA, Al-Dabet MM, Bock F, Nazir S, Huebner H, Mertens PR, Fischer KD, Zenclussen AC, Offermanns S, Aharon A, Brenner B, Shahzad K, Ruebner M, Isermann B (2016) Maternal extracellular vesicles and platelets promote preeclampsia via inflammasome activation in trophoblasts. Blood 128:2153–2164
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lam A, Fuller F, Miller J, Kloss J, Manthorpe M, Varon S, Cordell B (1991) Sequence and structural organization of the human gene encoding ciliary neurotrophic factor. Gene 102:271–276
Librach CL, Werb Z, Fitzgerald ML, Chiu K, Corwin NM, Esteves RA, Grobelny D, Galardy R, Damsky CH, Fisher SJ (1991) 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J Cell Biol 113:437–449
Mannelli C, Ietta F, Avanzati AM, Skarzynski D, Paulesu L (2015) Biological tools to study the effects of environmental contaminants at the feto-maternal interface. Dose Response 13:1559325815611902
Markert UR, Morales-Prieto DM, Fitzgerald JS (2011) Understanding the link between the IL-6 cytokine family and pregnancy: implications for future therapeutics. Expert Rev Clin Immunol 7:603–609
Meisser A, Cameo P, Islami D, Campana A, Bischof P (1999) Effects of interleukin-6 (IL-6) on cytotrophoblastic cells. Mol Hum Reprod 5:1055–1058
Moser G, Windsperger K, Pollheimer J, de Sousa Lopes SC, Huppertz B (2018) Human trophoblast invasion: new and unexpected routes and functions. Histochem Cell Biol 150:361–370
Muhlhauser J, Crescimanno C, Kasper M, Zaccheo D, Castellucci M (1995) Differentiation of human trophoblast populations involves alterations in cytokeratin patterns. J Histochem Cytochem 43:579–589
Nagase H, Visse R, Murphy G (2006) Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 69:562–573
Oefner CM, Sharkey A, Gardner L, Critchley H, Oyen M, Moffett A (2015) Collagen type IV at the fetal-maternal interface. Placenta 36:59–68
Perugini J, Di Mercurio E, Tossetta G, Severi I, Monaco F, Reguzzoni M, Tomasetti M, Dani C, Cinti S, Giordano A (2019) Biological effects of ciliary neurotrophic factor on hMADS adipocytes. Front Endocrinol (lausanne) 10:768
Rezende LF, Vieira AS, Negro A, Langone F, Boschero AC (2009) Ciliary neurotrophic factor (CNTF) signals through STAT3-SOCS3 pathway and protects rat pancreatic islets from cytokine-induced apoptosis. Cytokine 46:65–71
Rose-John S (2015) The soluble interleukin-6 receptor and related proteins. Best Pract Res Clin Endocrinol Metab 29:787–797
Sahay AS, Jadhav AT, Sundrani DP, Wagh GN, Mehendale SS, Joshi SR (2018) Matrix metalloproteinases-2 (MMP-2) and matrix metalloproteinases -9 (MMP-9) are differentially expressed in different regions of normal and preeclampsia placentae. J Cell Biochem 119:6657–6664
Sartor L, Negro A, Barletta E, Mugnai G, Garbisa S (2002) Modulation of proteolytic potential and differentiation by CNTF and BDNF in two mouse neuroblastoma clones: relation to invasion. Clin Exp Metastasis 19:709–716
Sati L, Demir AY, Sarikcioglu L, Demir R (2008) Arrangement of collagen fibers in human placental stem villi. Acta Histochem 110:371–379
Steinberg GR, Watt MJ, Ernst M, Birnbaum MJ, Kemp BE, Jorgensen SB (2009) Ciliary neurotrophic factor stimulates muscle glucose uptake by a PI3-kinase-dependent pathway that is impaired with obesity. Diabetes 58:829–839
Sundrani DP, Chavan-Gautam PM, Pisal HR, Mehendale SS, Joshi SR (2012) Matrix metalloproteinase-1 and -9 in human placenta during spontaneous vaginal delivery and caesarean sectioning in preterm pregnancy. PLoS ONE 7:e29855
Swierczewski A, Kobos J, Pasinski J, Kowalska-Koprek U, Karowicz-Bilinska A (2012) Expression of metalloproteinase MMP-9 and tissue inhibitor of metalloproteinase TIMP-2 in placenta of pregnant women with intrauterine growth restriction. Ginekol Pol 83:439–445
Szklanna PB, Wynne K, Nolan M, Egan K, Ainle FN, Maguire PB (2017) Comparative proteomic analysis of trophoblast cell models reveals their differential phenotypes, potential uses, and limitations. Proteomics 17:e1700037
Tossetta G, Fantone S, Giannubilo SR, Marinelli Busilacchi E, Ciavattini A, Castellucci M, Di Simone N, Mattioli-Belmonte M, Marzioni D (2019) Pre-eclampsia onset and SPARC: a possible involvement in placenta development. J Cell Physiol 234:6091–6098
Trimarchi T, Pachuau J, Shepherd A, Dey D, Martin-Caraballo M (2009) CNTF-evoked activation of JAK and ERK mediates the functional expression of T-type Ca2+ channels in chicken nodose neurons. J Neurochem 108:246–259
Vegh GL, Selcuk Tuncer Z, Fulop V, Genest DR, Mok SC, Berkowitz RS (1999) Matrix metalloproteinases and their inhibitors in gestational trophoblastic diseases and normal placenta. Gynecol Oncol 75:248–253
Wagener EM, Aurich M, Aparicio-Siegmund S, Floss DM, Garbers C, Breusing K, Rabe B, Schwanbeck R, Grotzinger J, Rose-John S, Scheller J (2014) The amino acid exchange R28E in ciliary neurotrophic factor (CNTF) abrogates interleukin-6 receptor-dependent but retains CNTF receptor-dependent signaling via glycoprotein 130 (gp130)/leukemia inhibitory factor receptor (LIFR). J Biol Chem 289:18442–18450
Wang Y, Fuller GM (1995) Interleukin-6 and ciliary neurotrophic factor trigger janus kinase activation and early gene response in rat hepatocytes. Gene 162:285–289
Weber M, Knoefler I, Schleussner E, Markert UR, Fitzgerald JS (2013) HTR8/SVneo cells display trophoblast progenitor cell-like characteristics indicative of self-renewal, repopulation activity, and expression of “stemness-” associated transcription factors. Biomed Res Int 2013:243649
Zong S, Li C, Luo C, Zhao X, Liu C, Wang K, Jia W, Bai M, Yin M, Bao S, Guo J, Kang J, Duan T, Zhou Q (2016) Dysregulated expression of IDO may cause unexplained recurrent spontaneous abortion through suppression of trophoblast cell proliferation and migration. Sci Rep 6:19916
Funding
Open access funding provided by Università Politecnica delle Marche within the CRUI-CARE Agreement. Funding for this work was provided by Scientific Research Grant from Università Politecnica delle Marche (2018–2020) to D.M., S.R.G. and A.G. Giovanni Tossetta is a recipient of a fellowship Starting Grant 2018 (SG-2018–12367994) of the Italian Ministry of Health.
Author information
Authors and Affiliations
Contributions
T. G., G.A. and M. D. contributed to the conception of the study. T.G., F.S., B.M.E., M.D. contributed to the design, performance, and data analysis of the experiments. G.S.R. and D.S.N. contributed to the sample collection. T.G., G.A. and M.D. contributed to the writing of the manuscript. All authors contributed to the critical revision and final approval of the article.
Corresponding authors
Ethics declarations
Ethics approval
The procedures followed for the collection of samples were in accordance with the Helsinki Declaration of 1975, as revised in 2013. The permission of the Human Investigation Committee of Marche Region (IT) was granted (protocol number 2019.172; study ID 980; CERM number 172).
Consent to participate
Pregnant women gave their written informed consent to the collection of placental specimens prior to inclusion in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The authors regret that Figure 2 showed in the article was the wrong one.
The original article has been corrected.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tossetta, G., Fantone, S., Busilacchi, E.M. et al. Modulation of matrix metalloproteases by ciliary neurotrophic factor in human placental development. Cell Tissue Res 390, 113–129 (2022). https://doi.org/10.1007/s00441-022-03658-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-022-03658-1