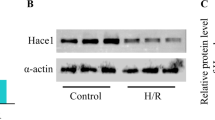
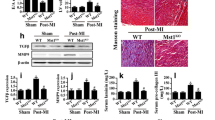
Abstract
Tripartite motif-containing protein (TRIM16) is a newly identified oxidative-stress-responsive protein. Oxidative stress is a hallmark of myocardial ischemia/reperfusion (I/R) injury and contributes to the cardiac injury. To date, whether TRIM16 plays a role in mediating oxidative stress during myocardial I/R injury is undetermined. The work is devoted to evaluate the possible relevance of TRIM16 in myocardial I/R injury. TRIM16 induction by myocardial hypoxia/reoxygenation (H/R) injury in vitro or myocardial I/R injury in vivo was observed. TRIM16 overexpression alleviated H/R-induced injury of rat cardiomyocytes. TRIM16 overexpression markedly attenuated cardiac injury, infarct size, and myocardial apoptosis induced by myocardial I/R injury. Further research revealed that TRIM16 was capable of enhancing Nrf2 activation via the regulation of Keap1. The inhibition of Nrf2 diminished TRIM16-overexpression-mediated cardioprotective effects. Overall, this work demonstrates that TRIM16 protects against myocardial I/R injury via affecting the Keap1/Nrf2 axis. This work offers new insights into the molecular mechanism underlying myocardial I/R injury and proposes TRIM16 as an attractive candidate target for cardioprotection.










Similar content being viewed by others
References
Beer HD, Munding C, Dubois N, Mamie C, Hohl D, Werner S (2002) The estrogen-responsive B box protein: a novel regulator of keratinocyte differentiation. J Biol Chem 277:20740–20749
Bell JL, Malyukova A, Holien JK, Koach J, Parker MW, Kavallaris M, Marshall GM and Cheung BB (2012) TRIM16 acts as an E3 ubiquitin ligase and can heterodimerize with other TRIM family members. PLoS One 7:e37470
Bell JL, Malyukova A, Kavallaris M, Marshall GM, Cheung BB (2013) TRIM16 inhibits neuroblastoma cell proliferation through cell cycle regulation and dynamic nuclear localization. Cell Cycle 12:889–898
Cheung BB, Bell J, Raif A, Bohlken A, Yan J, Roediger B, Poljak A, Smith S, Lee M, Thomas WD, Kavallaris M, Norris M, Haber M, Liu HL, Zajchowski D, Marshall GM (2006) The estrogen-responsive B box protein is a novel regulator of the retinoid signal. J Biol Chem 281:18246–18256
Frangogiannis NG (2015) Pathophysiology of Myocardial Infarction Compr Physiol 5:1841–1875
Hill MF, Singal PK (1996) Antioxidant and oxidative stress changes during heart failure subsequent to myocardial infarction in rats. Am J Pathol 148:291–300
Hirose K, Iwabuchi K, Shimada K, Kiyanagi T, Iwahara C, Nakayama H, Daida H (2011) Different responses to oxidized low-density lipoproteins in human polarized macrophages. Lipids Health Dis 10:1
Holmstrom KM, Finkel T (2014) Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol 15:411–421
Howden R (2013) Nrf2 and cardiovascular defense. Oxid Med Cell Longev 2013:104308
Huo X, Li S, Shi T, Suo A, Ruan Z, Yao Y (2015) Tripartite motif 16 inhibits epithelial-mesenchymal transition and metastasis by down-regulating sonic hedgehog pathway in non-small cell lung cancer cells. Biochem Biophys Res Commun 460:1021–1028
Jena KK, Kolapalli SP, Mehto S, Chauhan S and Chauhan S (2018) TRIM16 controls turnover of protein aggregates by modulating NRF2, ubiquitin system, and autophagy: implication for tumorigenesis. Mol Cell Oncol 5:e1532251
Jena KK, Kolapalli SP, Mehto S, Nath P, Das B, Sahoo PK, Ahad A, Syed GH, Raghav SK, Senapati S, Chauhan S and Chauhan S (2018) TRIM16 controls assembly and degradation of protein aggregates by modulating the p62-NRF2 axis and autophagy. EMBO J 37:e98358
Jena KK, Mehto S, Kolapalli SP, Nath P, Chauhan S, Chauhan S (2018c) TRIM16 employs NRF2, ubiquitin system and aggrephagy for safe disposal of stress-induced misfolded proteins. Cell Stress 2:365–367
Jena KK, Mehto S, Kolapalli SP, Nath P, Sahu R, Chauhan NR, Sahoo PK, Dhar K, Das SK, Chauhan S, Chauhan S (2019) TRIM16 governs the biogenesis and disposal of stress-induced protein aggregates to evade cytotoxicity: implication for neurodegeneration and cancer. Autophagy 15:924–926
Jiang B, Zhang B, Liang P, Chen G, Zhou B, Lv C, Tu Z, Xiao X (2013) Nucleolin protects the heart from ischaemia-reperfusion injury by up-regulating heat shock protein 32. Cardiovasc Res 99:92–101
Li L, Dong L, Qu X, Jin S, Lv X, Tan G (2016) Tripartite motif 16 inhibits hepatocellular carcinoma cell migration and invasion. Int J Oncol 48:1639–1649
Liu HL, Golder-Novoselsky E, Seto MH, Webster L, McClary J, Zajchowski DA (1998) The novel estrogen-responsive B-box protein (EBBP) gene is tamoxifen-regulated in cells expressing an estrogen receptor DNA-binding domain mutant. Mol Endocrinol 12:1733–1748
Lonborg JT (2015) Targeting reperfusion injury in the era of primary percutaneous coronary intervention: hope or hype? Heart 101:1612–1618
Munding C, Keller M, Niklaus G, Papin S, Tschopp J, Werner S, Beer HD (2006) The estrogen-responsive B box protein: a novel enhancer of interleukin-1beta secretion. Cell Death Differ 13:1938–1949
Nisole S, Stoye JP, Saib A (2005) TRIM family proteins: retroviral restriction and antiviral defence. Nat Rev Microbiol 3:799–808
Noguchi N (2008) Role of oxidative stress in adaptive responses in special reference to atherogenesis. J Clin Biochem Nutr 43:131–138
Python F, Goebel C, Aeby P (2009) Comparative DNA microarray analysis of human monocyte derived dendritic cells and MUTZ-3 cells exposed to the moderate skin sensitizer cinnamaldehyde. Toxicol Appl Pharmacol 239:273–283
Raif A, Marshall GM, Bell JL, Koach J, Tan O, D’Andreti C, Thomas W, Sekyere E, Norris M, Haber M, Kavallaris M, Cheung BB (2009) The estrogen-responsive B box protein (EBBP) restores retinoid sensitivity in retinoid-resistant cancer cells via effects on histone acetylation. Cancer Lett 277:82–90
Ren X, Yu J, Guo L and Ma H (2020) TRIM16 protects from OGD/R-induced oxidative stress in cultured hippocampal neurons by enhancing Nrf2/ARE antioxidant signaling via downregulation of Keap1. Exp Cell Res 391:111988
Shen Y, Liu X, Shi J, Wu X (2019) Involvement of Nrf2 in myocardial ischemia and reperfusion injury. Int J Biol Macromol 125:496–502
Silva-Islas CA, Maldonado PD (2018) Canonical and non-canonical mechanisms of Nrf2 activation. Pharmacol Res 134:92–99
Suzuki T, Motohashi H, Yamamoto M (2013) Toward clinical application of the Keap1-Nrf2 pathway. Trends Pharmacol Sci 34:340–346
Suzuki T, Yamamoto M (2015) Molecular basis of the Keap1-Nrf2 system. Free Radic Biol Med 88:93–100
Suzuki T, Yamamoto M (2017) Stress-sensing mechanisms and the physiological roles of the Keap1-Nrf2 system during cellular stress. J Biol Chem 292:16817–16824
Yao J, Xu T, Tian T, Fu X, Wang W, Li S, Shi T, Suo A, Ruan Z, Guo H, Yao Y (2016) Tripartite motif 16 suppresses breast cancer stem cell properties through regulation of Gli-1 degradation via the ubiquitin-proteasome pathway. Oncol Rep 35:1204–1212
Young IS, Woodside JV (2001) Antioxidants in health and disease. J Clin Pathol 54:176–186
Zhao Y, Liu H, Xi X, Chen S and Liu D (2020) TRIM16 protects human periodontal ligament stem cells from oxidative stress-induced damage via activation of PICOT. Exp Cell Res 397:112336
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Research involving human participants and/or animals
The animal use for the study was approved by the Ethics Committee of Shaanxi Provincial People’s Hospital, and the animal care and protocol were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85–23, revised 1996).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cui, Q., Yan, L. Tripartite motif-containing protein 16 protects against myocardial ischemia/reperfusion injury by affecting the Keap1/Nrf2 axis. Cell Tissue Res 386, 349–363 (2021). https://doi.org/10.1007/s00441-021-03518-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-021-03518-4