Abstract
Objective
Trimetazidine (TMZ) exerts a strong inhibitory effect on ischemia/reperfusion (I/R) injury. Inflammation plays a key role in I/R injury. We hypothesized that TMZ may protect cardiomyocytes from I/R injury by inhibiting inflammation.
Methods
The left anterior descending coronary artery was ligated for 30 min followed by 6 h of reperfusion to establish a model of I/R injury. H9c2 cardiomyocytes were subjected to 2 h of hypoxia and 3 h of normoxic conditions to establish a model of hypoxia/reoxygenation (H/R) injury. We monitored the change in pyroptosis by performing Western blot analysis, microscopy and ELISA.
Results
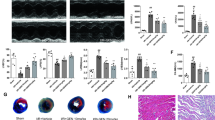
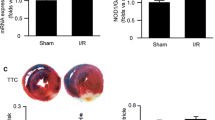
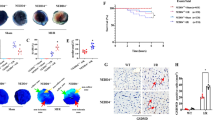
I/R and H/R treatment stimulated gasdermin D-N domain (GSDMD-N) expression in cardiomyocytes (sham onefold vs. I/R 2.5-fold; control onefold vs. H/R 2.0-fold). Moreover, TMZ increased the viability of H9c2 cardiomyocytes subjected to H/R treatment (H/R 65.0% vs. H/R + TMZ 85.3%) and reduced the infarct size in vivo (I/R 47.0% vs. I/R + TMZ 28.3%). H/R and I/R treatment increased the levels of TLR4, MyD88, phospho-NF-κB p65 and the NLRP3 inflammasome; however, TMZ reduced the expression of these proteins. Additionally, TMZ inhibited noncanonical inflammasome signaling induced by I/R injury.
Conclusions
In summary, TMZ alleviated pyroptosis induced by myocardial I/R injury through the TLR4/MyD88/NF-κB/NLRP3 inflammasome pathway. Therefore, TMZ represents an alternative treatment for myocardial I/R injury.








Similar content being viewed by others
Abbreviations
- AAR:
-
Area at risk
- CCK-8:
-
Cell counting kit-8
- CK-MB:
-
Creatine kinase-MB
- GSDMD-FL:
-
Gasdermin D-full length
- GSDMD-N:
-
Gasdermin D-N domain
- HE:
-
Hematoxylin and eosin
- H/R:
-
Hypoxia/reoxygenation
- INF:
-
Infarct size
- I/R:
-
Ischemia/reperfusion
- LAD:
-
Left anterior descending
- LDH:
-
Lactate dehydrogenase
- LV:
-
Left ventricle
- TMZ:
-
Trimetazidine
- TTC:
-
2,3,5-Triphenyltetrazolium chloride
References
Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart disease and stroke statistics-2018 update: a report from the American heart association. Circulation. 2018;137(12):e67–492.
Ibanez B, Heusch G, Ovize M, Van de Werf F. Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol. 2015;65(14):1454–71.
Hausenloy DJ, Yellon DM. Time to take myocardial reperfusion injury seriously. N Engl J Med. 2008;359(5):518–20.
Wu L, Tan J, Chen Z, Huang GJ. Cardioprotection of post-ischemic moderate ROS against ischemia/reperfusion via STAT3-induced the inhibition of MCU opening. Basic Res Cardiol. 2019;114(5):39.
Engler RL, Dahlgren MD, Morris DD, Peterson MA, Schmid-Schonbein GW. Role of leukocytes in response to acute myocardial ischemia and reflow in dogs. Am J Physiol. 1986;251(2 Pt 2):H314–23.
Suematsu M, DeLano F, Poole D, Engler R, Miyasaka M, Zweifach B, et al. Spatial and temporal correlation between leukocyte behavior and cell injury in postischemic rat skeletal muscle microcirculation. Lab Invest. 1994;70(5):684–95.
Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357(11):1121–35.
Ong SB, Hernandez-Resendiz S, Crespo-Avilan GE, Mukhametshina RT, Kwek XY, Cabrera-Fuentes HA, et al. Inflammation following acute myocardial infarction: Multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol Ther. 2018;186:73–87.
Arslan F, Smeets MB, O’Neill LA, Keogh B, McGuirk P, Timmers L, et al. Myocardial ischemia/reperfusion injury is mediated by leukocytic toll-like receptor-2 and reduced by systemic administration of a novel anti-toll-like receptor-2 antibody. Circulation. 2010;121(1):80–90.
Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9(3):113–4.
Wan P, Su W, Zhang Y, Li Z, Deng C, Li J, et al. LncRNA H19 initiates microglial pyroptosis and neuronal death in retinal ischemia/reperfusion injury. Cell Death Differ. 2020;27(1):176–91.
Sun R, Peng M, Xu P, Huang F, Xie Y, Li J, et al. Low-density lipoprotein receptor (LDLR) regulates NLRP3-mediated neuronal pyroptosis following cerebral ischemia/reperfusion injury. J Neuroinflammation. 2020;17(1):330.
Kantor PF, Lucien A, Kozak R, Lopaschuk GD. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res. 2000;86(5):580–8.
Wu S, Chang G, Gao L, Jiang D, Wang L, Li G, et al. Trimetazidine protects against myocardial ischemia/reperfusion injury by inhibiting excessive autophagy. J Mol Med (Berl). 2018;96(8):791–806.
Amini N, Sarkaki A, Dianat M, Mard S, Ahangarpour A, Badavi MJB, et al. The renoprotective effects of naringin and trimetazidine on renal ischemia/reperfusion injury in rats through inhibition of apoptosis and downregulation of micoRNA-10a. Biomed Pharmacother. 2019;112:108568.
Chen J, Wang B, Lai J, Braunstein Z, He M, Ruan G, et al. Trimetazidine attenuates cardiac dysfunction in endotoxemia and sepsis by promoting neutrophil migration. Front Immunol. 2018;9:2015.
Kuzmicic J, Parra V, Verdejo HE, Lopez-Crisosto C, Chiong M, Garcia L, et al. Trimetazidine prevents palmitate-induced mitochondrial fission and dysfunction in cultured cardiomyocytes. Biochem Pharmacol. 2014;91(3):323–36.
Kolik LG, Nadorova AV, Stolyaruk VN, Miroshkina IA, Tsorin IB, Kryzhanovskii SA. Anxiolytic properties of trimetazidine in experimental models of increased anxiety. Bull Exp Biol Med. 2017;162(5):643–6.
Jorgensen I, Miao EA. Pyroptotic cell death defends against intracellular pathogens. Immunol Rev. 2015;265(1):130–42.
Woodall MC, Woodall BP, Gao E, Yuan A, Koch WJ. Cardiac fibroblast GRK2 deletion enhances contractility and remodeling following ischemia/reperfusion injury. Circ Res. 2016;119(10):1116–27.
Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117(14):3720–32.
Toldo S, Schatz AM, Mezzaroma E, Chawla R, Stallard TW, Stallard WC, et al. Recombinant human interleukin-1 receptor antagonist provides cardioprotection during myocardial ischemia reperfusion in the mouse. Cardiovasc Drugs Ther. 2012;26(3):273–6.
Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM, Nunez G. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38(6):1142–53.
Compan V, Baroja-Mazo A, Lopez-Castejon G, Gomez AI, Martinez CM, Angosto D, et al. Cell volume regulation modulates NLRP3 inflammasome activation. Immunity. 2012;37(3):487–500.
Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36(3):401–14.
Ives A, Nomura J, Martinon F, Roger T, LeRoy D, Miner JN, et al. Xanthine oxidoreductase regulates macrophage IL1beta secretion upon NLRP3 inflammasome activation. Nat Commun. 2015;6:6555.
Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535(7610):111–6.
Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8(11):1812–25.
Shi J, Gao W, Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42(4):245–54.
Grebe A, Hoss F, Latz E. NLRP3 Inflammasome and the IL-1 pathway in atherosclerosis. Circ Res. 2018;122(12):1722–40.
Xu B, Jiang M, Chu Y, Wang W, Chen D, Li X, et al. Gasdermin D plays a key role as a pyroptosis executor of non-alcoholic steatohepatitis in humans and mice. J Hepatol. 2018;68(4):773–82.
Kang R, Zeng L, Zhu S, Xie Y, Liu J, Wen Q, et al. Lipid peroxidation drives gasdermin D-mediated pyroptosis in lethal polymicrobial sepsis. Cell Host Microbe. 2018;24(1):97-108e4.
Zhang Z, Shao X, Jiang N, Mou S, Gu L, Li S, et al. Caspase-11-mediated tubular epithelial pyroptosis underlies contrast-induced acute kidney injury. Cell Death Dis. 2018;9(10):983.
Hua S, Ma M, Fei X, Zhang Y, Gong F, Fang M. Glycyrrhizin attenuates hepatic ischemia-reperfusion injury by suppressing HMGB1-dependent GSDMD-mediated Kupffer cells pyroptosis. Int Immunopharmacol. 2019;68:145–55.
Zhang D, Qian J, Zhang P, Li H, Shen H, Li X, et al. Gasdermin D serves as a key executioner of pyroptosis in experimental cerebral ischemia and reperfusion model both in vivo and in vitro. J Neurosci Res. 2019;97(6):645–60.
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–5.
Kovacs SB, Miao EA. Gasdermins: effectors of pyroptosis. Trends Cell Biol. 2017;27(9):673–84.
Pateras I, Giaginis C, Tsigris C, Patsouris E, Theocharis S. NF-kappaB signaling at the crossroads of inflammation and atherogenesis: searching for new therapeutic links. Expert Opin Ther Targets. 2014;18(9):1089–101.
Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526(7575):666–71.
Diao C, Chen Z, Qiu T, Liu H, Yang Y, Liu X, et al. Inhibition of PRMT5 attenuates oxidative stress-induced pyroptosis via activation of the Nrf2/HO-1 signal pathway in a mouse model of renal ischemia-reperfusion injury. Oxid Med Cell Longev. 2019;2019:2345658.
Ghoneim M, Abdallah D, Shebl A, El-Abhar HJT. The interrupted cross-talk of inflammatory and oxidative stress trajectories signifies the effect of artesunate against hepatic ischemia/reperfusion-induced inflammasomopathy. Toxicol Appl Pharmacol. 2020;409:115.
Okondo M, Johnson D, Sridharan R, Go E, Chui A, Wang M, et al. DPP8 and DPP9 inhibition induces pro-caspase-1-dependent monocyte and macrophage pyroptosis. Nat Chem Biol. 2017;13(1):46–53.
She Y, Shao L, Zhang Y, Hao Y, Cai Y, Cheng Z, et al. Neuroprotective effect of glycosides in Buyang Huanwu Decoction on pyroptosis following cerebral ischemia-reperfusion injury in rats. J Ethnopharmacol. 2019;242:112051.
Wang Y, Yan X, Mi S, Li Z, Wang Y, Zhu H, et al. Naoxintong attenuates Ischaemia/reperfusion Injury through inhibiting NLRP3 inflammasome activation. J Cell Mol Med. 2017;21(1):4–12.
Hauet T, Goujon J, Vandewalle A, Baumert H, Lacoste L, Tillement J, et al. Trimetazidine reduces renal dysfunction by limiting the cold ischemia/reperfusion injury in autotransplanted pig kidneys. J Am Soc Nephrol. 2000;11(1):138–48.
Amber KI, Hadi NR, Muhammad-Baqir BM, Jamil DA, Al-Aubaidy HA. Trimetazidine attenuates the acute inflammatory response induced by Novolimus eluting bioresorbable coronary scaffold implantation. Int J Cardiol. 2016;220:514–9.
Zhou X, Li C, Xu W, Chen J. Trimetazidine protects against smoking-induced left ventricular remodeling via attenuating oxidative stress, apoptosis, and inflammation. PLoS ONE. 2012;7(7): e40424.
Zhang H, Liu M, Zhang Y, Li XJ. Trimetazidine Attenuates Exhaustive Exercise-Induced Myocardial Injury in Rats via Regulation of the Nrf2/NF-κB Signaling Pathway. Front Pharmacol. 2019;10:175.
Wan P, Su W, Zhang Y, Li Z, Deng C, Zhuo Y. Trimetazidine protects retinal ganglion cells from acute glaucoma via the Nrf2/Ho-1 pathway. Clin Sci. 2017;131(18):2363–75.
Acknowledgements
The work was supported by the National Natural Science Foundation of China (Grant No. 81670227 and 81600341), the Natural Science Foundation of Zhejiang Province (Grant No. LQ19H020004), the Cardiac Rehabilitation and Metabolic Therapy research fund (Grant No. 2019ZJXQN03), the Medical Health Science and Technology Project of Zhejiang Provincial (Grant No. 2021KY1072), the Wenzhou Science and Technology Bureau (Grant No. Y20170045, Y20160030 and Y20180105) and the Traditional Chinese Medicine Administration of Zhejiang Province (Grant No. 2016ZA137).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11_2021_1530_MOESM1_ESM.tif
Supplementary file1 (TIF 1362 KB) Supplementary Fig. S1 Schematic of the protocol and structure of TMZ. (A) Schematic of the protocol for the I/R and H/R study. After different treatments, tissue or cell samples were collected for further experimentation. (B) Schematic representation of the groups and indicators. (C) The structure of TMZ used in this study. I/R, ischemia/reperfusion; TMZ, trimetazidine; control, normoxic; H/R, hypoxia/reoxygenation; CK-MB, creatine kinase-MB; TTC, 2,3,5-triphenyltetrazolium chloride; HE, hematoxylin-eosin; IHC, immunohistochemistry; WB, Western blot analysis; ELISA, enzyme-linked immunosorbent assay; LAD, left anterior descending; CCK-8, Cell Counting Kit-8; LDH, lactate dehydrogenase; IF, immunofluorescence
Rights and permissions
About this article
Cite this article
Chen, X., Lin, S., Dai, S. et al. Trimetazidine affects pyroptosis by targeting GSDMD in myocardial ischemia/reperfusion injury. Inflamm. Res. 71, 227–241 (2022). https://doi.org/10.1007/s00011-021-01530-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-021-01530-6