Abstract
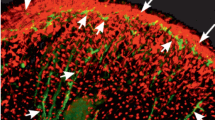
FMRFamide-related peptides (FaRPs) are a class of neuropeptides that participate in a variety of physiological processes in invertebrates. They occur in nerves of stomatogastric ganglia and enteroendocrine cells of the insect digestive tract, where they may control muscle functions. However, their direct involvement in muscle function has never been shown in situ. We studied the relationship between FaRPs and midgut muscle during larval–pupal transition of the mosquito Aedes aegypti. In late L4, FaRP-positive neuronal extensions attach to the bundles of the external circular muscle layer, and muscle stem cells start to undergo mitosis in the internal circular layer. Thereafter, the external muscle layer degenerates, disappearing during early pupal development, and is completely absent in the adult mosquito. Our results indicate that FaRP-based neural signals are involved in the reorganization of the muscle fibers of the mosquito midgut during the larval–pupal transition. In addition to confirming FaRP involvement in muscle function, we show that the mosquito midgut muscles are largely innervated, and that circular and longitudinal muscle have specific neuron bodies associated with them.








Similar content being viewed by others
References
Aghajanian P, Takashima S, Paul M, Younossi-Hartenstein A, Hartenstein V (2016) Metamorphosis of the Drosophila visceral musculature and its role in intestinal morphogenesis and stem cell formation. J Development 420:43–59
Beehler-Evans R, Micchelli CA (2015) Generation of enteroendocrine cell diversity in midgut stem cell lineages. J Development 142:654–664
Bernick EP, Moffett SB, Moffett DF (2007) Organization, ultrastructure, and development of midgut visceral muscle in larval Aedes aegypti. J Tissue Cell 39:277–292
Bernick EP, Moffett SB, Moffett DF (2008) Ultrastructure and morphology of midgut visceral muscle in early pupal Aedes aegypti mosquitoes. J Tissue Cell 40:127–141
Brown MR, Crim JW, Lea AO (1986) FMRFamide-and pancreatic polypeptidelike immunoreactivity of endocrine cells in the midgut of a mosquito. J Tissue Cell 18:419–428
Brown MR, Lea A (1988) FMRFamide-and adipokinetic hormone-Like immunoreactivity in the nervous system of the mosquito, Aedes aegypti. J Comp Neurol 614:606–614
Brown MR, Klowden MJ, Crim JW, Young L, Shrouder LA, Lea AO (1994) Endogenous regulation of mosquito host-seeking behavior by a neuropeptide. J Insect Physiol 40:399–406
Bureau I (2015) Novel neuropeptides useful as insecticides Field. The present invention relates to peptide fragments and analogs derived from background of the invention neuropeptides play a key role in the regulation of a variety of physiological’, Israel Patente WO 2015/052701
Copenhaver PF (2007) How to innervate a simple gut: familiar themes and unique aspects in the formation of the insect enteric nervous system. J Dev Dyn 236:1841–1864
Cottrell GA (1993) The wide range of actions of the FMRFamide-related peptides and the biological importance of peptidergic messengers. Comparative Molecular Neurobiology, Birkhäuser Basel 279–285
Dowling JE (1991) Dopamine release from interplexiform cells in the retina: effects of bicuculline, and enkephalin on horizontal. J Neurosci 11:3034–3046
Duttlinger A, Berry K, Nichols R (2002) The different effects of three Drosophila melanogaster dFMRFamide-containing peptides on crop contractions suggest these structurally related peptides do not play redundant functions in gut. J Peptides 23:1953–1957
Elphick MR, Mirabeau O (2014) The evolution and variety of RFamide-type neuropeptides: insights from deuterostomian invertebrates. J Front Endocrinol 5:93
Fadda M, Hasakiogullari I, Temmerman L, Beets I, Zels S, Schoofs L (2019) Regulation of feeding and metabolism by neuropeptide F and short neuropeptide F in invertebrates. Front Endocrinol 10:64
Fernandes KM, Neves CA, Serrão JE, Martins GF (2014) Aedes aegypti midgut remodeling during metamorphosis. Parasitol Int 63:506–512
Fónagy A (2007) Insect neuropeptides and their potential application for pest control. Acta phytopathologica et entomologica Hungarica 41:137–152
Godoy RSM, Fernandes KM, Martins GF (2015) Midgut of the non-hematophagous mosquito Toxorhynchites theobaldi (Diptera, Culicidae). Sci Rep 5:1–16
Gonzalez-Gaitan M, Jackle H (1995) Invagination centers within the Drosophila stomatogastric nervous system anlage are positioned by Notch-mediated signaling which is spatially controlled through wingless. J Development 121:2313–2325
Hartenstein V (1997) Development of the insect stomatogastric nervous system. J Trends Neurosci 20:421–427
Hao K, Ullah H, Jarwar AR, Nong X, Tu X, Zhang Z (2020) Functional identification of an FMRFamide-related peptide gene on diapause induction of the migratory locust, Locusta migratoria L. Genomics 112:1821–1828
Haselton AT, Yin CM, Stoffolano JG (2008) FMRFamide-like immunoreactivity in the central nervous system and alimentary tract of the non-hematophagous blow fly, Phormia regina, and the hematophagous horse fly, Tabanus nigrovittatus. J Insect Sci 8:1–17
Hill SR, Orchard I (2004) The influence of diet and feeding state on FMRFamide-related peptides in the gut of Locusta migratoria L. J Peptides 25:105–114
Hung RJ, Hu Y, Kirchner R, Liu Y, Xu C, Comjean A, Perrimon N (2020) A cell atlas of the adult Drosophila midgut. Proc Natl Acad Sci 117:1514–1523
Jones JC, Zeve VH (2003) The fine structure of the gastric caeca of Aedes aegypti larvae. J Insect Physiol 14:1567–1575
Kingan TG, Shabanowitz J, Hunt D, Witten J (1996) Characterization of two myotrophic neuropeptides in the FMRFamide family from the segmental ganglia of the moth Manduca sexta: candidate neurohormones and neuromodulators. J Exp Biol 199:1095–1104
Klapper R (2000) The longitudinal visceral musculature of Drosophila melanogaster persists through metamorphosis. J Mech Dev 95:47–54
Krajniak KG (2013) Invertebrate FMRFamide Related Peptides. J Prot Pept Lett 20:647–670
Lee JS, Shih PY, Schaedel ON, Quintero-Cadena P, Rogers AK, Sternberg PW (2017) FMRFamide-like peptides expand the behavioral repertoire of a densely connected nervous system. J Proc Natl Acad Sci 114:E10726–E10735
Lenaerts C, Cools D, Verdonck R, Verbakel L, Broeck JV, Marchal E (2017) The ecdysis triggering hormone system is essential for successful moulting of a major hemimetabolous pest insect, Schistocerca gregaria. Sci Rep 7:1–14
López-Vera E, Aguilar MB, Heimer de la Cotera EP (2008) FMRFamide and related peptides in the phylum mollusca. Peptides 29:310–317
Martin BS, Gomez MR, Landgraf M, Bate M (2001) A distinct set of founders and fusion-competent myoblasts make visceral muscles in the Drosophila embryo. J Development 128:3331–3338
McLachlan IG, Beets I, Bono M, Heiman MG (2018) A neuronal MAP kinase constrains growth of a Caenorhabditis elegans sensory dendrite throughout the life of the organism. J PLoS Gen 14:1–22
Micchelli CA, Sudmeier L, Perrimon N, Tang S, Beehler-Evans R (2011) Identification of adult midgut precursors in Drosophila. J Gene Exp Patt 11:12–21
Milakovic M, Ormerod KG, Klose MK, Mercier AJ (2014) Mode of action of a Drosophila FMRFamide in inducing muscle contraction. J Exp Biol 217:1725–1736
Moffett SB, Moffett DF (2014) Comparison of immunoreactivity to serotonin, FMRFamide and SCPb in the gut and visceral nervous system of larvae, pupae and adults of the yellow fever mosquito Aedes aegypti. J Insect Sci 5:20
Nagata S (2016) FMRFamides. In: Handbook of hormones. Academic Press 432–433
Nässel DR, Winther ÅM (2010) Drosophila neuropeptides in regulation of physiology and behavior. Prog Neurobiol 92:42–104
Nässel DR, Zandawala M (2019) Recent advances in neuropeptide signaling in Drosophila, from genes to physiology and behavior. Prog Neurobiol 179:101607
Nassel RD, Bayraktaroglu E, Dircksen H (1994) Neuropeptides in neurosecretory and efferent neural systems of insect thoracic and abdominal ganglia. J Zool Sci 11:15–31
Nichols R, McCormick J, Lim I (1999) Regulation of Drosophila FMRFamide neuropeptide gene expression. J Neurobiology 39:347–358
Nishiura JT, Ho P, Ray K (2003) Methoprene interferes with mosquito midgut remodeling during metamorphosis. J Med Entomol 40:498–507
Nishiura JT, Ray K, Murray J (2005) Expression of nuclear receptor-transcription factor genes during Aedes aegypti midgut metamorphosis and the effect of methoprene on expression. Insect Biochem Mol Biol 35:561–573
Oliveira AH, Gonçalves WG, Fernandes KM, Barcellos MS, Sampaio WMS, Lopes MP, Martins GF, Serrão JE (2019) Morphology and morphometry of the midgut in the stingless bee Friesella schrottkyi (Hymenoptera: Apidae). J Insects 10:73
Onken H, Moffett SB, Moffett DF (2004) The anterior stomach of larval mosquitoes (Aedes aegypti): effects of neuropeptides on transepithelial ion transport and muscular motility. J Exp Biol 207:3731–3739
Orchard I, Lange AB, Bendena WG (2001) FMRFamide-related peptides: a multifunctional family of structurally related neuropeptides in insects. Adv Insect Physiol 28:267–329
Parthasarathy R, Palli SR (2007) Stage-and cell-specific expression of ecdysone receptors and ecdysone-induced transcription factors during midgut remodeling in the yellow fever mosquito, Aedes aegypti. J Insect Physiol 53:216–229
Peters RS, Meusemann K, Petersen M, Mayer C, Wilbrandt J, Ziesmann T, Donath A, Kjer KM, Aspöck U, Aspöck H (2014) The evolutionary history of holometabolous insects inferred from transcriptome-based phylogeny and comprehensive morphological data. J BMC Evol Biol 14:52
Predel R, Neupert S, Garczynski SF, Crim JW, Brown MR, Russell WK, Nachman RJ (2010) Neuropeptidomics of the mosquito Aedes aegypti. J Proteome Res 9:2006–2015
Price DA, Greenberg MJ (1977a) Purification characterization of a cardioexcitatory neuropeptide from the central ganglia of a bivalve mollusc. J Prep Bioch 7:261–281
Price DA, Greenberg MJ (1977b) Structure of a molluscan cardioexcitatory neuropeptide. J Science 197:670–671
Price DA, Greenberg MJ (2007) The Hunting of the FaRPs: The distribution of FMRFamide-related peptides. J Biol Bull 177:198–205
Reiher W, Shirras C, Kahnt J, Baumeister S, Isaac RE, Wegener C (2011) Peptidomics and peptide hormone processing in the Drosophila midgut. J Proteome Res 10:1881–1892
Roller L, Čižmár D, Gáliková Z, Bednár B, Daubnerová I, Žitňan D (2016) Molecular cloning, expression and identification of the promoter regulatory region for the neuropeptide trissin in the nervous system of the silkmoth Bombyx mori. Cell Tissue Res 364:499–512
Rudolf A, Buttgereit D, Jacobs M, Wolfstetter G, Kesper D, Pütz M, Berger S, Renkawitz-Pohl R, Holz A, Önel SF (2014) Distinct genetic programs guide Drosophila circular and longitudinal visceral myoblast fusion. J BMC Cell Biol 15:27
Scherkenbeck J, Zdobinsky T (2009) Insect neuropeptides: structures, chemical modifications and potential for insect control. J Bioorg Med Chem 17:4071–4084
Schinkmann K, Li C (1992) Localization of FMRFamide-like peptides in Caenorhabditis elegans. J Compar Neurol 316:251–260
Sedra L, Lange AB (2016) Cloning and expression of long neuropeptide F and the role of FMRFamide-like peptides in regulating egg production in the Chagas vector, Rhodnius prolixus. Peptides 82:1–11
Seron TJ, Hill J, Linser PJ (2004) A GPI-linked carbonic anhydrase expressed in the larval mosquito midgut. J Exp Biol 207:4559–4572
Souza DLL, Zanuncio JC, Serrão JE (2016) FMRFamide-cells in the midgut of Scaptotrigona xanthotricha (Apidae: Meliponini) of different ages and fed different diets. J Apicul Research 55:428–432
Stanek DM, Pohl J, Crim JW, Brown MR (2002) Neuropeptide F and its expression in the yellow fever mosquito, Aedes aegypti. Peptides 23:1367–1378
Strand MR, Brown MR, Vogel KJ (2016) Mosquito peptide hormones: diversity, production, and function. Adv Insect Physiol 51:145–188
Stracker TH, Thompson S, Grossman GL, Riehle MA, Brown MR (2002) Characterization of the AeaHP gene and its expression in the mosquito Aedes aegypti (Diptera: Culicidae). J Med Entomol 39:331–342
Taghert PH, Nitabach MN (2012) Peptide neuromodulation in invertebrate model systems. Neuron 76:82–97
Tarr EA, Fidler BM, Gee KE, Anderson CM, Jager AK, Gallagher NM, Fabian-Fine R (2019) Distribution of FMRFamide-related peptides and co-localization with glutamate in Cupiennius salei, an invertebrate model system. Cell Tissue Res 376:83–96
Tune TC, Irving T, Sponberg S (2020) Nanometer-scale structure differences in the myofilament lattice spacing of two two cockroach leg muscles correspond to their different functions. J Exp Biol 9:223
Tuthill JC, Wilson RI (2016a) Mechanosensation and adaptive motor control in insects. J Curr Biol 26:R1022–R1038
Tuthill JC, Wilson RI (2016b) Parallel transformation of tactile signals in central circuits of Drosophila. J Cell 164:1046–1059
Veenstra JA, Lau GW, Agricola HJ, Petzel DH (1995) Immunohistological localization of regulatory peptides in the midgut of the female mosquitoAedes aegypti. Histochemistry and Cell Biology 104:337–347
Warr E, Aguilar R, Dong Y, Mahairaki V, Dimopoulos G (2007) Spatial and sex-specific dissection of the Anopheles gambiae midgut transcriptome. BMC Genomics 8:1–11
Weetman D, Kamgang B, Badolo A, Moyes CL, Shearer FM, Coulibaly M, Pinto J, Lambrechts L, McCall PJ (2018) Aedes mosquitoes and Aedes-borne arboviruses in Africa: current and future threats. Int J Environ Res Public Health 15:220
Wikgren M, Fagerholm HP (1993) Neuropeptides in sensory structures of nematodes. J Acta Biol Hung 44:133–136
Wu Y, Parthasarathy R, Bai H, Palli SR (2006) Mechanisms of midgut remodeling: juvenile hormone analog methoprene blocks midgut metamorphosis by modulating ecdysone action. Mech Dev 123:530–547
Xu Y, Cleary L, Byrne J (2018) Identification and characterization of pleural neurons that inhibit tail sensory neurons and motor neurons in Aplysia: correlation with FMRFamide immunoreactivity. J Neuroscience 14:3565–3577
Zels S, Dillen S, Crabbé K, Spit J, Nachman RJ, Broeck JV (2015) Sulfakinin is an important regulator of digestive processes in the migratory locust, Locusta migratoria. Insect Biochem Mol Biol 61:8–16
Zhang W, Yan Z, Li B, Jan LY, Jan YN (2014) Identification of motor neurons and a mechanosensitive sensory neuron in the defecation circuitry of Drosophila larvae. J eLife 3:e03293
Žitňan D, Šauman I, Sehnal F (1993) Peptidergic innervation and endocrine cells of insect midgut. J Arch Insect Bioch Physiol 22:113–132
Acknowledgements
We thank the Núcleo de Microscopia e Microanálise (NMM, UFV) for letting us use the confocal microscope. We are grateful to Nágila Francinete Costa Secundino and Paulo Fillemon Paolucci Pimenta for their support with the reagents and use of scanning electron microscopes at the Instituto René Rachou, Fiocruz, MG.
Funding
This work was supported by the Universidade Federal de Viçosa, Comissão de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)—support to RSMG; the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (Fapemig; APQ-00560–17)—support to GFM, and by the National Institutes of Health (USA) R01AI031478 and the Bloomberg Philanthropies to MJL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study was performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and the Animal Use Manual (FIOCRUZ, Ministry of Health of Brazil, national decree, no. 3179). The protocol was approved by the Ethics Committee of Universidade Federal de Viçosa (UFV-Protocol 561/2016).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Movie S1 Specificity of the FaRP immunoreactivity (green) in the midgut of late L4 of Aedes aegypti. The grid formed by the external circular muscles (ECMs) fused with the longitudinal muscles (LMs) are shown in a more external position in relation to the midgut epithelium; therefore, they are seen first in the z-stack video. The internal muscle (ICM) layer is more internal and shown above the ECM/LM grid. The FaRP immunoreactivity is inside ring-shaped structures attached to the mid-position of the ECM between two cruciform points (fusion regions of ECM and LM). (MOV 1129 KB)
Supplementary file2 Movie S2 Muscle framework (red) of the anterior midgut of early L4 of Aedes aegypti. The external circular muscle (ECM) layer is shown. (MP4 253 KB)
Supplementary file3 Movie S3. Muscle framework (red) of the anterior midgut of the early pupal Aedes aegypti. The external circular muscle (ECM) layer in the process of degeneration is shown. (MP4 424 KB)
Supplementary file4 Movie S4 Mitotic nuclei (green) in the internal circular muscle layer (ICM) of the early pupal midgut of Aedes aegypti. All labeled nuclei were found in the ICM bundles. No mitosis labeling was observed in the midgut epithelium. Red: midgut muscles; blue: cell nuclei. (MOV 626 KB)
Supplementary file5 Movie S5 Muscle framework (red) of the anterior midgut of adult female Aedes aegypti. The external circular muscle (ECM) layer is not present. (MP4 344 KB)
441_2021_3462_MOESM7_ESM.tiff
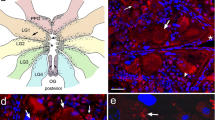
Supplementary file7 Fig. S1 FaRP and PH3 immunolabeling, neurite and neuron body identifications in immature stages of Aedes aegypti; and the absence of ECM layer in the adult mosquito. (a) FaRP-positive (green) small, rounded points (white arrow) in the posterior midgut of Aedes aegypti early L4. ICM: internal circular muscle layer. Red: midgut muscles; Blue: cell nuclei. (b) Mitotic nuclei (green) of regenerative cells (RCs) from the midgut epithelium in the early L4 of A. aegypti. DC: digestive cell nuclei. (c) 3D view of the FaRP immunoreactivity (green) in the grid formed by the presence of an external circular muscle (ECM) layer fused with the longitudinal muscle (LM) layers in the L4 of A. aegypti. Ring-shaped structures (arrow) are shown in the mid-position of the ECM just between two cruciform points (*) (where ECM fuses with LM). The internal circular muscle (ICM) fibers are shown between two ECM bundles. Red: midgut muscles. (d) Scanning electron microscopy (SEM) of the ring-shaped structures (white arrows) attached to the mid-position of the ECM between two cruciform points (*). Their extensions are also shown (black arrow). (e) FaRP-positive (green) small, rounded points (arrow) lined up and in close association with LM bundles in the anterior midgut of A. aegypti early pupa. (f–h) SEM of the early pupal (f) and early L4 (g, h) midgut, showing the region where the ICM bifurcates (full arrow), close to the two prominent longitudinal muscles (pLMs). (f) In the early pupa, it is difficult to identify the internal neurons using SEM, however, the predicted regions of their presence are shown with the thin arrows. (g, h) In the late L4, both bifurcations of the ICM (full arrow) and the internal neurons (thin arrows) are identified in g. Details of the ICM neurons are shown in h. (i) Midgut muscle organization (red) in the anterior midgut of the adult female of A. aegypti. The pLM; LM; and ICM bundles and their bifurcations (full arrow) are detected in a similar configuration of the larval midgut; however, the ECM is not present (no grids of cruciform regions are detected) (TIFF 5705 KB)
Rights and permissions
About this article
Cite this article
Godoy, R.S.M., Barbosa, R.C., Procópio, T.F. et al. FMRF-related peptides in Aedes aegypti midgut: neuromuscular connections and enteric nervous system. Cell Tissue Res 385, 585–602 (2021). https://doi.org/10.1007/s00441-021-03462-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-021-03462-3