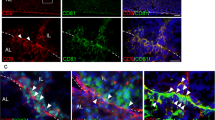
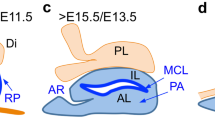
Abstract
A supply of hormone-producing cells from stem/progenitor cells is critical to sustain the endocrine activity of the pituitary gland. In the adenohypophysis composing the anterior and intermediate lobe (AL and IL, respectively), stem/progenitor cells expressing sex-determining region Y-box 2 (SOX2) and S100β are located in the marginal cell layer (MCL) facing Rathke’s cleft (primary niche) and the parenchyma of the AL (secondary niche). Our previous studies using mice and rats indicated that the tetraspanin superfamily CD9 and CD81 are expressed in S100β/SOX2-positive cells of primary and secondary niches (named CD9/CD81/S100β/SOX2-positive cell), and the cells located in the AL-side niches exhibit plasticity and multipotency. However, it is unclear whether CD9/CD81/S100β/SOX2-positive cells in the IL-side primary niche are stem/progenitor cells for the AL or IL. Here, we successfully isolated pure CD9/CD81/S100β/SOX2-positive cells from the IL-side primary niche. They had a higher level of S100β and SOX2 mRNA and a greater pituisphere forming capacity than those of CD9/CD81/S100β/SOX2-positive cells isolated from the AL. They also had capacity to differentiate into all types of adenohypophyseal hormone-producing cells, concomitantly with the loss of CD9 expression. Loss of CD9 and CD81 function in CD9/CD81/S100β/SOX2-positive cells by siRNA treatment impaired prolactin cell differentiation. Consistently, in the pituitary gland of CD9/CD81 double knockout mice, dysgenesis of the MCL and a lower population of prolactin cells were observed. These results suggest that the CD9/CD81/S100β/SOX2-positive cells in the MCL of the IL-side are potential suppliers of adult core stem cells in the AL.







Similar content being viewed by others
References
Boucheix C, Rubinstein E (2001) Tetraspanins. Cell Mol Life Sci 58:1189–1205
Chen J, Hersmus N, Van Duppen V, Caesens P, Denef C, Vankelecom H (2005) The adult pituitary contains a cell population displaying stem/progenitor cell and early embryonic characteristics. Endocrinol 146:3985–3998
Fauquier T, Rizzoti K, Dattani M, Lovell-Badge R, Robinson IC (2008) SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc Natl Acad Sci USA 105:2907–2912
Gremeaux L, Fu Q, Chen J, Vankelecom H (2012) Activated phenotype of the pituitary stem/progenitor cell compartment during the early-postnatal maturation phase of the gland. Stem Cells Dev 21:801–813
Hemler ME (2005) Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol 6:801–811
Horiguchi K, Fujiwara K, Kouki T, Kikuchi M, Yashiro T (2008) Immunohistochemistry of connexin 43 throughout anterior pituitary gland in a transgenic rat with green fluorescent protein-expressing folliculo-stellate cells. Anat Sci Int 83:256–260
Horiguchi K, Fujiwara K, Yoshida S, Higuchi M, Tsukada T, Kanno N, Yashiro T, Tateno K, Osako S, Kato T, Kato Y (2014) Isolation of dendritic-cell-like S100beta-positive cells in rat anterior pituitary gland. Cell Tissue Res 357:301–308
Horiguchi K, Fujiwara K, Yoshida S, Nakakura T, Arae K, Tsukada T, Hasegawa R, Takigami S, Ohsako S, Yashiro T, Kato T, Kato Y (2018) Isolation and characterisation of CD9-positive pituitary adult stem/progenitor cells in rats. Sci Rep 8:5533
Horiguchi K, Fujiwara K, Yoshida S, Tsukada T, Hasegawa R, Takigami S, Ohsako S, Yashiro T, Kato T, Kato Y (2020a) CX3CL1/CX3CR1-signalling in the CD9/S100beta/SOX2-positive adult pituitary stem/progenitor cells modulates differentiation into endothelial cells. Histochem Cell Biol 153:385–396
Horiguchi K, Nakakura T, Yoshida S, Tsukada T, Kanno N, Hasegawa R, Takigami S, Ohsako S, Kato T, Kato Y (2016) Identification of THY1 as a novel thyrotrope marker and THY1 antibody-mediated thyrotrope isolation in the rat anterior pituitary gland. Biochem Biophys Res Commun 480:273–279
Horiguchi K, Yoshida S, Tsukada T, Nakakura T, Fujiwara K, Hasegawa R, Takigami S, Ohsako S (2020b) Expression and functions of cluster of differentiation 9 and 81 in rat mammary epithelial cells. J Reprod Dev 66:515–522
Japon MA, Rubinstein M, Low MJ (1994) In situ hybridization analysis of anterior pituitary hormone gene expression during fetal mouse development. J Histochem Cytochem 42:1117–1125
Jin Y, Takeda Y, Kondo Y, Tripathi LP, Kang S, Takeshita H, Kuhara H, Maeda Y, Higashiguchi M, Miyake K, Morimura O, Koba T, Hayama Y, Koyama S, Nakanishi K, Iwasaki T, Tetsumoto S, Tsujino K, Kuroyama M, Iwahori K, Hirata H, Takimoto T, Suzuki M, Nagatomo I, Sugimoto K, Fujii Y, Kida H, Mizuguchi K, Ito M, Kijima T, Rakugi H, Mekada E, Tachibana I, Kumanogoh A (2018) Double deletion of tetraspanins CD9 and CD81 in mice leads to a syndrome resembling accelerated aging. Sci Rep 8:5145
Matsubara M, Harigaya T, Nogami H (2001) Effects of diethylstilbestrol on the cytogenesis of prolactin cells in the pars distalis of the pituitary gland of the mouse. Cell Tissue Res 306:301–307
Ogasawara K, Nogami H, Tsuda MC, Gustafsson JA, Korach KS, Ogawa S, Harigaya T, Hisano S (2009) Hormonal regulation of prolactin cell development in the fetal pituitary gland of the mouse. Endocrinol 150:1061–1068
Oomizu S, Takeuchi S, Takahashi S (1998) Stimulatory effect of insulin-like growth factor I on proliferation of mouse pituitary cells in serum-free culture. J Endocrinol 157:53–62
Sato K, Watanabe YG (1998) Corticosteroids stimulate the differentiation of growth hormone cells but suppress that of prolactin cells in the fetal rat pituitary. Arch Histol Cytol 61:75–81
Suga H, Kadoshima T, Minaguchi M, Ohgushi M, Soen M, Nakano T, Takata N, Wataya T, Muguruma K, Miyoshi H, Yonemura S, Oiso Y, Sasai Y (2011) Self-formation of functional adenohypophysis in three-dimensional culture. Nature 480:57–62
Scully KM, Gleiberman AS, Lindzey J, Lubahn DB, Korach KS, Rosenfeld MG (1997) Role of estrogen receptor-alpha in the anterior pituitary gland. Mol Endocrinol 11:674–681
Stefaneanu L, Powell-Braxton L, Won W, Chandrashekar V, Bartke A (1999) Somatotroph and lactotroph changes in the adenohypophyses of mice with disrupted insulin-like growth factor I gene. Endocrinol 140:3881–3889
Vankelecom H, Chen J (2014) Pituitary stem cells: where do we stand? Mol Cell Endocrinol 385:2–17
Yoshida S, Kato T, Kato Y (2016a) Regulatory System for Stem/Progenitor Cell Niches in the Adult Rodent Pituitary. Int J Mol Sci 17:75
Yoshida S, Nishimura N, Ueharu H, Kanno N, Higuchi M, Horiguchi K, Kato T, Kato Y (2016b) Isolation of adult pituitary stem/progenitor cell clusters located in the parenchyma of the rat anterior lobe. Stem Cell Res 17:318–329
Acknowledgements
We are grateful to Dr. T. Kato and Y. Kato (Institute for Reproduction and Endocrinology, Meiji University) for helpful discussions. We thank the “Joint Usage/Research Center for Endocrine/Metabolism, Institute for Molecular and Cellular Regulation, Gunma University” (www.imcr.gunma-u.ac.jp/activity/activity3) for providing antibodies.
Funding
This work was supported by JSPS KAKENHI Grants (nos. 19K07255 to K.H. and nos. 17K08517 to K.F.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The current study was approved by the Committee on Animal Experiments of the School of Agriculture, Meiji University, and Kyorin University based on the NIH Guidelines for the Care and Use of Laboratory Animals. This article does not contain any studies with human participants. This article does not contain any studies with human participants performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
441_2021_3460_MOESM1_ESM.tif
Supplementary file1 (TIF 6011 KB) Supplementary Fig. 1. Differentiation capacity of pituispheres formed using CD9-positive cells of the AL (a) Double immunofluorescence staining for CD9 (red) and SOX2 (green) in the 2nd passage of a pituisphere formed by AL-side CD9-positive cells. (b) DAPI staining (blue) and double immunofluorescence staining for CD9 (red) and pituitary hormones (GH, PRL, LHβ, TSHβ, ACTH) or S100β in a pituisphere (green). (c) Proportions of each hormone-positive cell among pituispheres differentiated from AL-side CD9-positive cells. (d) mRNA levels of the indicated genes in pituispheres of AL-side CD9-positive cells before or after differentiation induction, determined by qPCR (mean ± SEM, n = 6), followed by normalisation with an internal control (Actb): Cd9, Cd81, S100β, Sox2, Pit1, Gh, Prl, Tshβ, LHβ, and Pomc. **P < 0.01. *P < 0.05. Scale bars, 50 μm.
441_2021_3460_MOESM2_ESM.tif
Supplementary file2 (TIF 8927 KB) Supplementary Fig. 2. Differentiation capacity of pituispheres formed by CD9-positive cells of the AL after Cd9 and Cd81 siRNA treatment. (a) mRNA levels of Cd9, Cd81, S100β, and Sox2 in CD9-positive cells after Cd9 and Cd81 siRNA treatment, determined by qPCR (mean ± SEM, n = 6), followed by normalisation with an internal control (Actb). **P < 0.01. (b) Double immunofluorescence staining for CD9 (red) and CD81 (green) in a pituisphere treated with siRNAs. (c) Bright images of a pituisphere of AL-side CD9-positive cells after siRNA treatment. (d) The number of pituispheres formed by AL-side CD9-positive cells after treatment with non-silencing siRNA and Cd9/Cd81 siRNAs. (e) mRNA levels of Cd9, Cd81, S100β, and Sox2 in pituispheres formed by AL-side CD9-positive cells after siRNA treatment, determined by qPCR (mean ± SEM, n = 6), followed by normalisation with an internal control (Actb). **P < 0.01. (f) Double immunofluorescence staining for PRL and GH in an siRNA-treated pituisphere (left: non-silencing siRNA, right: Cd9/Cd81 siRNA). (g) GH and PRL cell populations after differentiation induction in pituispheres formed by AL-side CD9-positive cells after siRNA treatment. (h) mRNA levels of Sox2, Erα, and Prl in pituispheres formed by AL-side CD9-positive cells after siRNA treatment in the presence/absence of DES, determined by qPCR (mean ± SEM, n = 4), followed by normalisation with an internal control (Actb). **P < 0.01. Scale bars, 50 μm.
441_2021_3460_MOESM3_ESM.tif
Supplementary file3 (TIF 22201 KB Supplementary Fig. 3. Immunostaining for anterior pituitary hormones in paraffin sections of WT and CD9/CD81 DKO mice. (a and b) 1st panel: HE staining. 2nd–6th panels: Immunostaining for PRL, GH, TSHβ, LHβ, and ACTH in the pituitary of the WT (a) and CD9/CD81 DKO mice (b). Right panels show high magnification of the boxed area in each left panel. (c) Immunofluorescence staining for S100β in paraffin sections of WT (Left panel) and CD9/CD81 DKO mice (Right panel). DAPI staining (blue) and immunofluorescence staining for S100β (green). AL, anterior lobe; IL, intermediate lobe; PL, posterior lobe; RC, Rathke’s cleft; MCL, marginal cell layer. III; third ventricle. Scale bars, 200 μm (a, b, and c, left panels), 20 μm (a and b, right panels).)
Rights and permissions
About this article
Cite this article
Horiguchi, K., Fujiwara, K., Takeda, Y. et al. CD9-positive cells in the intermediate lobe of the pituitary gland are important supplier for prolactin-producing cells in the anterior lobe. Cell Tissue Res 385, 713–726 (2021). https://doi.org/10.1007/s00441-021-03460-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-021-03460-5