Abstract
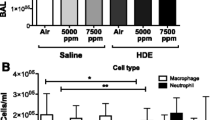
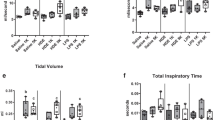
Animal production units produce and store many contaminants on-site, including organic dust (OD) and hydrogen sulfide (H2S). Workers in these settings report various respiratory disease symptoms. Both OD and H2S have shown to induce lung inflammation. However, impact of co-exposure to both H2S and OD has not been investigated. Therefore, we tested a hypothesis that pre-exposure to H2S modulates the innate inflammatory response of the lungs to organic dust. In a mouse model of H2S and organic dust extract (ODE) exposure, we assessed lung inflammation quantitatively. We exposed human airway epithelial and monocytic cells to medium or H2S alone or H2S followed by ODE and measured cell viability, oxidative stress, and other markers of inflammation. Exposure to 10 ppm H2S followed by ODE increased the lavage fluid leukocytes. However, exposure to 10 ppm H2S alone resulted in changes in tight junction proteins, an increase in mRNA levels of tlr2 and tlr4 as well as ncf1, ncf4, hif1α, and nrf2. H2S alone or H2S and ODE exposure decreased cell viability and increased reactive nitrogen species production. ODE exposure increased the transcripts of tlr2 and tlr4 in both in vitro and in vivo models, whereas increased nfkbp65 transcripts following exposure to ODE and H2S was seen only in in vitro model. H2S alone and H2S followed by ODE exposure increased the levels of IL-1β. We conclude that pre-exposure to H2S modulates lung innate inflammatory response to ODE.












Similar content being viewed by others
References
American Conference of Governmental Industrial Hygienists (2019) Monitoring H2S to meet new exposure standards—Occupational Health & Safety. vol 2019. Occupational Health and Safety, ohsonline.com
American Thoracic Society (1998) Respiratory health hazards in agriculture. Am J Respir Crit Care Med 158:S1–S76
Anantharam P, Kim DS, Whitley EM et al (2018) Midazolam efficacy against acute hydrogen sulfide-induced mortality and neurotoxicity. J Med Toxicol 14:79–90
Anantharam P, Whitley EM, Mahama B et al (2017a) Characterizing a mouse model for evaluation of countermeasures against hydrogen sulfide-induced neurotoxicity and neurological sequelae. Ann N Y Acad Sci 1400:46–64
Anantharam P, Whitley EM, Mahama B et al (2017b) Cobinamide is effective for treatment of hydrogen sulfide-induced neurological sequelae in a mouse model. Ann N Y Acad Sci 1408:61–78
Basic A, Alizadehgharib S, Dahlén G et al (2017) Hydrogen sulfide exposure induces NLRP3 inflammasome-dependent IL-1β and IL-18 secretion in human mononuclear leukocytes in vitro. Clin Exp Dent Res 3:115–120
Bhat SM, Massey N, Karriker LA, Singh B, Charavaryamath C (2019) Ethyl pyruvate reduces organic dust-induced airway inflammation by targeting HMGB1-RAGE signaling. Respir Res 20:27
Bosshart H, Heinzelmann M (2016) THP-1 cells as a model for human monocytes. Ann Transl Med 4:438–438
Charavaryamath C, Juneau V, Suri SS et al (2008) Role of toll-like receptor 4 in lung inflammation following exposure to swine barn air. Exp Lung Res 34:19–35
Charavaryamath C, Singh B (2006) Pulmonary effects of exposure to pig barn air. J Occup Med Toxicol 1:10
Che J, Yue D, Zhang B et al (2018) Claudin-3 inhibits lung squamous cell carcinoma cell epithelial-mesenchymal transition and invasion via suppression of the wnt/beta-catenin signaling pathway. Int J Med Sci 15:339–351
Chen YH, Wang PP, Wang XM et al (2011) Involvement of endogenous hydrogen sulfide in cigarette smoke-induced changes in airway responsiveness and inflammation of rat lung. Cytokine 53:334–341
Chen YH, Wu R, Geng B et al (2009) Endogenous hydrogen sulfide reduces airway inflammation and remodeling in a rat model of asthma. Cytokine 45:117–123
Christiani DC (1996) Organic dust exposure and chronic airway disease. Am J Respir Crit Care Med 154:833–834
Chung KF (2014) Hydrogen sulfide as a potential biomarker of asthma. Expert Rev Respir Med 8:5–13
Costigan MG (2003) Hydrogen sulfide: UK occupational exposure limits. Occup Environ Med 60:308–312
de Souza WF, Fortunato-Miranda N, Robbs BK et al (2013) Claudin-3 overexpression increases the malignant potential of colorectal cancer cells: roles of ERK1/2 and PI3K-Akt as modulators of EGFR signaling. PLoS ONE 8:e74994
Esechie A, Kiss L, Olah G et al (2008) Protective effect of hydrogen sulfide in a murine model of acute lung injury induced by combined burn and smoke inhalation. Clin Sci (Lond) 115:91–97
Gerald CL, McClendon CJ, Ranabhat RS et al (2019) Sorrel extract reduces oxidant production in airway epithelial cells exposed to swine barn dust extract in vitro. Mediators Inflamm 2019:7420468
Giuffre A, Vicente JB (2018) Hydrogen sulfide biochemistry and interplay with other gaseous mediators in mammalian physiology. Oxid Med Cell Longev 2018:6290931
Gordon R, Hogan CE, Neal ML et al (2011) A simple magnetic separation method for high-yield isolation of pure primary microglia. J Neurosci Methods 194:287–296
Han W, Dong Z, Dimitropoulou C et al (2011) Hydrogen sulfide ameliorates tobacco smoke-induced oxidative stress and emphysema in mice. Antioxid Redox Signal 15:2121–2134
Hine C, Harputlugil E, Zhang Y et al (2015) Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell 160:132–144
Hu L-F, Wong PT-H, Moore PK et al (2007) Hydrogen sulfide attenuates lipopolysaccharide-induced inflammation by inhibition of p38 mitogen-activated protein kinase in microglia. J Neurochem 100:1121–1128
Iowa State University and University of Iowa (2002) IOWA CONCENTRATED ANIMAL FEEDING OPERATIONS AIR QUALITY STUDY. Final Report., vol 2018. Iowa State University and University of Iowa, Iowa
Jiang J, Chan A, Ali S et al (2016) Hydrogen sulfide-mechanisms of toxicity and development of an antidote. Sci Rep 6:20831–20831
Kim DS, Anantharam P, Hoffmann A et al (2018) Broad spectrum proteomics analysis of the inferior colliculus following acute hydrogen sulfide exposure. Toxicol Appl Pharmacol 355:28–42
Kotton DN (2018) Claudin-18: unexpected regulator of lung alveolar epithelial cell proliferation. J Clin Invest 128:903–905
Legator MS, Singleton CR, Morris DL et al (2001) Health effects from chronic low-level exposure to hydrogen sulfide. Archives of Environmental Health: An International Journal 56:123–131
Lewis RJ, Copley GB (2015) Chronic low-level hydrogen sulfide exposure and potential effects on human health: a review of the epidemiological evidence. Crit Rev Toxicol 45:93–123
Li L, Rose P, Moore PK (2011) Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol 51:169–187
Linden DR, Levitt MD, Farrugia G et al (2010) Endogenous production of H2S in the gastrointestinal tract: still in search of a physiologic function. Antioxid Redox Signal 12:1135–1146
Massey N, Puttachary S, Mahadev-Bhat S et al (2019) HMGB1-RAGE Signaling plays a role in organic dust-induced microglial activation and neuroinflammation. Toxicol Sci 169(2):579–592
McGovern T, Farahnak S, Chen M, Larsson K, Martin JG, Adner M (2019) Organic dust, causing both oxidative stress and Nrf2 activation, is phagocytized by bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 317:L305–l316
Nath Neerukonda S, Mahadev-Bhat S, Aylward B et al (2018) Kinome analyses of inflammatory responses to swine barn dust extract in human bronchial epithelial and monocyte cell lines. Innate Immun 24:366–381
Ni J-Q, Heber AJ, Diehl CA et al (2000) SE—structures and environment: ammonia, hydrogen sulphide and carbon dioxide release from pig manure in under-floor deep pits. J Agric Eng Res 77:53–66
Nordgren TM, Charavaryamath C (2018) Agriculture occupational exposures and factors affecting health effects. Curr Allergy Asthma Rep 18:65
Oesterhelweg L, Puschel K (2008) Death may come on like a stroke of lightening”: phenomenological and morphological aspects of fatalities caused by manure gas. Int J Legal Med 122:101–107
Oh GS, Pae HO, Lee BS et al (2006) Hydrogen sulfide inhibits nitric oxide production and nuclear factor-kappaB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radic Biol Med 41:106–119
Park JH, Peters TM, Altmaier R et al (2013) Simulation of air quality and cost to ventilate swine farrowing facilities in winter. Comput Electron Agric 98:136–145
Pavilonis BT, O’Shaughnessy PT, Altmaier R et al (2013) Passive monitors to measure hydrogen sulfide near concentrated animal feeding operations. Environ Sci Process Impacts 15:1271–1278
Pender RL, Minor RC, Hurley SL et al (2014) Exposure to swine housing dust modulates macrophage morphology and function. Am J Immunol 10:35–45
Poole JA, Wyatt TA, Kielian T et al (2011) Toll-like receptor 2 regulates organic dust-induced airway inflammation. Am J Respir Cell Mol Biol 45:711–719
Poole JA, Wyatt TA, Oldenburg PJ et al (2009) Intranasal organic dust exposure-induced airway adaptation response marked by persistent lung inflammation and pathology in mice. Am J Physiol Lung Cell Mol Physiol 296:L1085-1095
Rao X, Huang X, Zhou Z et al (2013) An improvement of the 2ˆ(–delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostatistics, bioinformatics and biomathematics 3:71–85
Richardson DB (1995) Respiratory effects of chronic hydrogen sulfide exposure. Am J Ind Med 28:99–108
Romberger DJ, Bodlak V, Von Essen SG et al (1985) Wyatt TA (2002) Hog barn dust extract stimulates IL-8 and IL-6 release in human bronchial epithelial cells via PKC activation. J Appl Physiol (1985) 93:289–296
Sethi RS, Schneberger D, Charavaryamath C et al (2017) Pulmonary innate inflammatory responses to agricultural occupational contaminants. Cell Tissue Res 367:1–16
Shi C, Pamer EG (2011) Monocyte recruitment during infection and inflammation. Nat Rev Immunol 11:762–774
Spassov SG, Donus R, Ihle PM et al (2017) Hydrogen sulfide prevents formation of reactive oxygen species through PI3K/Akt signaling and limits ventilator-induced lung injury. Oxid Med Cell Longev 2017:3715037
Tamizhselvi R, Moore PK, Bhatia M (2008) Inhibition of hydrogen sulfide synthesis attenuates chemokine production and protects mice against acute pancreatitis and associated lung injury. Pancreas 36:e24-31
The National Institutes for Occupational Safety and Health (NIOSH) (2018) CDC - Immediately Dangerous to Life or Health Concentrations (IDLH): hydrogen sulfide - NIOSH Publications and Products. vol 2020. The Centers for Disease Control and Prevention
Trevisani M, Patacchini R, Nicoletti P et al (2005) Hydrogen sulfide causes vanilloid receptor 1-mediated neurogenic inflammation in the airways. Br J Pharmacol 145:1123–1131
Vested A, Basinas I, Burdorf A et al (2019) A nationwide follow-up study of occupational organic dust exposure and risk of chronic obstructive pulmonary disease (COPD). Occup Environ Med 76:105–113
Wang X, Gray Z, Willette-Brown J et al (2018) Macrophage inducible nitric oxide synthase circulates inflammation and promotes lung carcinogenesis. Cell Death Discovery 4:46
Willis WL, Wang L, Wada TT et al (2018) The proinflammatory protein HMGB1 is a substrate of transglutaminase-2 and forms high-molecular weight complexes with autoantigens. J Biol Chem 293(22):8394–8409
Zhang G, Wang P, Yang G et al (2013) The inhibitory role of hydrogen sulfide in airway hyperresponsiveness and inflammation in a mouse model of asthma. Am J Pathol 182:1188–1195
Zhang JY, Ding YP, Wang Z et al (2017) Hydrogen sulfide therapy in brain diseases: from bench to bedside. Med Gas Res 7:113–119
Zhang P, Li F, Wiegman CH et al (2015) Inhibitory effect of hydrogen sulfide on ozone-induced airway inflammation, oxidative stress, and bronchial hyperresponsiveness. Am J Respir Cell Mol Biol 52:129–137
Zhao Y, Biggs TD, Xian M (2014) Hydrogen sulfide (H2S) releasing agents: chemistry and biological applications. Chem Commun (Camb) 50:11788–11805
Zhi L, Ang AD, Zhang H et al (2007) Hydrogen sulfide induces the synthesis of proinflammatory cytokines in human monocyte cell line U937 via the ERK-NF-kappaB pathway. J Leukoc Biol 81:1322–1332
Acknowledgments
We would like to thank Dr. Locke A. Karriker (VDPAM, Iowa State University) for supplying us with OD samples. We would like to thank Dr. Jacek Koziel (Department of Agricultural and Biosystems Engineering, Iowa State University) for initial help with the measurement of H2S concentrations and the Department of Biomedical Sciences for providing access to the core laboratory facilities.
Funding
C.C. laboratory is currently funded through a startup grant from Iowa State University and seed grants from the College of Veterinary Medicine, Iowa State University. This manuscript was supported in part by Grant Number 5 U54 OH007548 from CDC—NIOSH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Since this manuscript does not contain any studies with human participants, it is not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
441_2020_3333_MOESM1_ESM.tif
Supplementary file1: Fig. S1 H2S and ODE exposure induced histopathological changes in the lungs H&E images are shown in the left and inflammatory score is shown in the right. Histopathological changes due to exposure to either H2S or ODE or co-exposure to H2S and ODE were not significantly different among any of the groups. Compared with controls (a-c), ODE exposure of mice did not induce any morphological changes in the blood vessels, bronchioles and septa except for the number of club cells (b). (Micrometer bar = 100 µm). Data (mean ± SEM, n = 5–6/group) analyzed with one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons as compared with 0 ppm (TIF 92 MB)
Rights and permissions
About this article
Cite this article
Shrestha, D., Bhat, S.M., Massey, N. et al. Pre-exposure to hydrogen sulfide modulates the innate inflammatory response to organic dust. Cell Tissue Res 384, 129–148 (2021). https://doi.org/10.1007/s00441-020-03333-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-020-03333-3