Abstract
In the molecular biological and ultrastructural studies of the peritubular wall cells encasing the seminiferous tubules of mammalian testes, we found it necessary to characterize the outermost cell layer bordering on the interstitial space in detail. For half a century, the extremely thin cells of this monolayer have in the literature been regarded as part of a lymphatic endothelium, in particular in rodents. However, our double-label immunofluorescence microscopical results have shown that in all six mammalian species examined, including three rodent ones (rat, mouse, guinea pig), this classification is not correct: the very attenuated cells of this monolayer are not of lymphatic endothelial nature as they do not contain established endothelial marker molecules. In particular, they do not contain claudin-5-positive tight junctions, VE-cadherin-positive adherens junctions, “lymph vessel endothelium hyaluronan receptor 1” (LYVE-1), podoplanin, protein myozap and “von Willebrand Factor” (vWF). By contrast and as controls, all these established marker molecules for the lymphatic endothelial cell type are found in the endothelia of the lymph and—partly also—blood vessels located nearby in the interstitial space. Thus, our results provide evidence that the monolayer cells covering the peritubular wall do not contain endothelial marker molecules and hence are not endothelial cells. We discuss possible methodological reasons for the maintenance of this incorrect cell type classification in the literature and emphasize the value of molecular analyses using multiple cell type–specific markers, also with respect to physiology and medical sciences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Historically, cell types and tissues have long been given names based on morphological or putative functional aspects or with the use of the name of the assumed discoverer. In the original microscopical studies of testicular tissues, the peritubular wall (PW) cells encasing the seminiferous tubules (STs) have been given a variety of terminological names of which “myoid” has apparently been the mostly used one (for a recent report showing that these cells even represent cells of a novel kind of smooth muscle (SM) tissue, the “lamellar smooth muscle cells” (LSMCs), see Domke and Franke 2019). However, very early on a special exception was introduced and has been repetitively asserted in the literature: the outermost monolayer of very thin and broad cells covering the PW and bordering on the interstitial space has been widely referred to—in particular for rodent testes—as part of a “lymphatic endothelium,” i.e., an endothelial cell layer, thus contributing to the formation of a special category of “lymphatic vessels” (e.g., Fawcett et al. 1969, 1970, 1973; Dym and Fawcett 1970; Dym 1975, 1988, 1994; Clark 1976; Connell 1976; Wrobel et al. 1979, 1981; Söderström 1981; Hadley and Dym 1987; Maekawa et al. 1996; Yazama et al. 1997; Losinno et al. 2012, 2016). To demonstrate the constant reiteration and wide distribution of this concept of a lymphatic endothelial cell layer covering the PW, we present a historical selection list of 15 examples in a Supplementary Literature and Document Collection (SLDC; Online Resource 1). This classification as “lymphatic endothelium” has also special weight as the supporters include some of the widely known lymphatic vessel experts (e.g., Leak and Burke 1968; Fawcett et al. 1969, 1970; Leak 1970, 1971, 1976; Holstein et al. 1979; Weiss 1983). Only very few authors have named these cells “fibrocytes” or “fibroblasts” but also without any specific structural or molecular evidence (e.g., Bressler and Ross 1972). To clarify this cell type, we felt obliged to examine and characterize the cells of this layer using established markers for the lymphatic endothelial cell type.
Material and Methods
Tissues and antibodies
Testes of animals of six mammalian species (man, bull, boar, rat, mouse and guinea pig) were used as described for snap-frozen tissue samples or chemically fixed tissues in previous publications (see, e.g., Domke et al. 2014; Domke 2018; Domke and Franke 2019). For controls, the following tissues were used in parallel: STs and testicular excurrent ducts, liver, intestine, tongue mucosa, esophagus, heart and bladder.
Antibodies used for immunoblot analyses of polypeptides separated by gel electrophoresis as well as for immunofluorescence microscopy are listed in Tables 1 and 2 of Domke and Franke (2019) as well as in Table 6 of Domke (2018). Antibodies used in particular for the identification or exclusion of possible endothelial marker molecules and special other typical endothelial components are listed in Table 1 of our present article. For the antibody binding methods applied, see also Domke et al. (2014), Domke (2018) and Domke and Franke (2019). For certain aspects, we have also used serial ultrathin sections in tomography analyses.
Results
Electron microscopy
In all species studied, the STs (seminiferous tubules; tubuli seminiferi) were tightly encased by the PW structure consisting of monolayers of polyhedral, rather flat LSMCs, on both sides associated with layers of extracellular matrix (ECM) structures, dominated by collagen filament bundles oriented parallel to the specific ST (denoted C in Fig. 1a, d). In the different species examined, the number of such LSMC monolayers varies between one to five or six (in rodents, mostly one). In general, this PW appears to be surrounded by an additional, very attenuated monolayer of polyhedral cells in which SM structures are usually not detectable. Figures 1, 2, and 3 present in cross-sections the electron microscopical appearance of PW structures in different mammalian species (Fig. 1a: bovine testis; for an example of an anomalously thin boar testis PW with only one to two LSMC layers, see Figs. 1b, 2 and 3 rodents).
Electron micrographs of ultrathin cross-sections through the peripheral regions of seminiferous tubules (ST) of bull (a, b, c) and boar (d) testes, showing the peritubular wall (PW) and its lamellar smooth muscle cell (LSMC) layers (nos. 1–6 in a; denoted by brackets in d), interspersed with layers of collagen (C)-rich extracellular matrix (ECM) the first of which is attached to the basal lamina (BL; denoted by arrowheads in d) associated with the Sertoli cells (SC). Note that in such sections, the cells of the outermost layers are very attenuated (labelled nos. 5 and 6 in a and denoted by arrows in d; see also b and c) revealing a very wide lamellar or very thin filopodial shape. For special details of the latter see the insert figures b and c in which such tubular or filopodial cell protrusions are denoted by arrowheads; the arrow in c denotes a very thin intracytoplasmic membrane-surrounded structure containing extremely small vesicles. N, nucleus. Bars 1 μm (a, d), 500 nm (b, c)
Electron micrographs of ultrathin sections through the outer attenuated lamellar cells of PWs surrounding STs of rat testes, showing the specific monolayers of the very thin outermost cells (arrows), followed by a layer of ECM material, a single monolayer of LSMCs (brackets; note the densely packed myofilament bundles) and another ECM layer associated with the basal lamina and Sertoli cells (SC). Note the very large and very attenuated outermost cell layer regions and their locally variable protrusions into the interstitial space. N, nucleus. Bars 1 μm
Electron micrographs of ultrathin sections through mouse testicular tissue, showing details of the LSMC monolayers (brackets) and the thin cell outermost monolayers (arrows) of the PW encasing the STs (SC, Sertoli cell). Note also the interspersed, relatively thin ECM layers and the basal lamina associated with the Sertoli cells (denoted by arrowheads). Note also the two forms of the thin lamellar cells, i.e., the LSMCs and the very attenuated outermost monolayer cells with regions protruding into the interstitial space. Bars 1 μm
In all species examined, the cells of this outermost layer are extremely flat and broad, down to 10–25-nm inner membrane-to-membrane distance (see, e.g., Fig. 1b), i.e., in large regions even more attenuated than the LSMC regions described in our recent paper (Domke and Franke 2019). In Figs. 2 and 3, this is specifically shown for testes of two rodent species, i.e., rat and mouse. Here, the outermost cell layer shows remarkable regional differences of the course and ECM material associations. In some regions, it is rather closely associated with the subjacent ECM layer but there are also regions in which variously sized parts of the uppermost layer are widely dissociated from the subjacent ECM and protrude into the interstitial space (e.g., Figs. 2c–e and 3c–e). From our electron microscopic studies, it is also clear that rather large regions of this outermost cell layer are not stably attached to an ECM structure and also do not bear a distinct apical extracellular glycocalyx (Figs. 1, 2, and 3). Electron microscopy of serial ultrathin sections has further shown that these outermost, very attenuated cells commonly do not contain caveolae structures and that their basal plasma membrane is not attached to other cells or ECM structures via tangles of “anchoring filaments,” i.e., two ultrastructural criteria characteristic for several types of lymphatic vessels (for various subtypes and for lymphangiogenesis, see, e.g., Leak and Burke 1968; Leak 1970, 1976; Daróczy 1988; Jussila and Alitalo 2002; Luttun et al. 2004; Baluk and McDonald 2008; Karpanen and Alitalo 2008; Bruyère and Noël 2010).
Double-label immunofluorescence microscopy
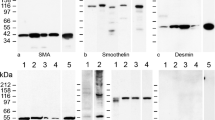
When cryostat sections through testicular tissues of bulls, boars, or men were immunostained for two different specific antigens, their entire PWs were intensely positive for SM cell type marker molecules such as desmin, SM α-actin and smoothelin as were the vascular SM walls, in some places also for vimentin (for some examples see, e.g., Figs. 4a–d and 5; see also Domke and Franke 2019). By contrast, the endothelial structures of all testicular blood and lymph vessels, located nearby in the interstitial space, were the only positive cells for the specific endothelial marker molecules used such as claudin-5, VE-cadherinFootnote 1, protein LYVE-1 and podoplanin (Figs. 4, 5, and 6) as well as protein myozap and von Willebrand factor VIII. These lymphatic endothelia were mostly very thin or appeared—in grazing sections—as extended structures (e.g., Figs. 4a and 5a). In cross-sections through rather small lymphatic vessels, near-colocalizations were seen for different endothelial junction markers that are known to be closely located to each other (see, e.g., Fig. 5b–b‴ for claudin-5 and VE-cadherin in yellow merger color), in certain lymphatic endothelial regions even “completely” colocalizing (see, e.g., also Baluk et al. 2007). No immunostaining of endothelial markers was seen along—and in—cells of the specific outermost monolayer of the PWs (for an example of more widely reacting molecules see below). This general difference of intense SM protein positivity in PW cells on the one hand and distinct endothelium-specific marker labelling of vascular endothelia on the other was especially striking in some sections through porcine testes that showed a very high frequency of blood as well as lymph vessels in the interstitial space (Fig. 6).
Double-label immunofluorescence microscopy of cryostat cross-sections through STs of frozen bull (a–c) and human (d) testes. Immunostaining reactions are shown with antibodies against the smooth muscle (SM) cell markers desmin (a; green) and smooth muscle α-actin (α-SMA, b–d; green), in comparison with the endothelial cell markers claudin-5 (a; red) and VE-cadherin (b–d; red). The peritubular LSMCs as well as the cells of the blood vessel (V) walls in the interstitial space (I) are positive for the SMC markers but negative for the blood and lymph vessel endothelial markers that, on the other hand, are positive on all vascular endothelial cells, including compact and small SMC-free small lymphatic vessels (some are denoted by arrows). Note that the entire peritubular LSMC walls on their outer wall show an absence of the endothelial marker reaction. L, lumen. Bars 20 μm
Double-label immunofluorescence microscopy of cryostat cross-sections through STs of frozen human (a, a′) and bovine (b, b‴) testes (a′ and b‴ present the specific reactions on a phase contrast background). Immunostaining reactions of peritubular LSMCs (brackets) as well as blood vessel (V) walls are shown for the SMC marker smooth muscle α-actin (α-SMA, a, a′; green). In comparison, the vascular endothelial junction markers LYVE-1 (a, a′; red), claudin-5 (b, b″, b‴; red) and VE-cadherin (b′, b‴; green) react with the endothelium of the blood vessels (V) and lymph vessels (red contours and punctate reactions). Note that even the smallest lymphatic vessels are positive for the endothelial markers but that Sertoli and spermatogonial cells are totally negative for both. Note also the near-colocalization of both endothelial junction markers. L, lumen; I, interstitial space. Bars 50 μm
Double-label immunofluorescence microscopy of a cryostat cross-section through STs of frozen boar testis, showing immunostaining with antibodies against VE-cadherin (red) and smooth muscle α-actin (α-SMA; green). The vascular endothelial marker again visualizes specifically cell–cell adherens junctions (AJs) of the numerous blood vessels (some are denoted by V) and the endothelial cell structures of the numerous small lymphatic vessels (purely red structures). Peritubular LSMC walls (denoted by brackets) and vascular walls are totally negative for VE-cadherin but totally positive for α-SMA. L, lumen; I, interstitial space. Bar 50 μm
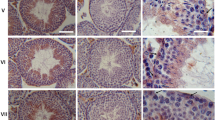
The same pattern of immunostaining results has been found for all three rodent species examined. Essentially no endothelial marker reactions have been detected in the outermost layers of the PWs but only in the separate—generally much smaller—lymph and blood vessels in the interstitial space (Fig. 7 presents examples of rat testis after reactions with claudin-5 and VE-cadherin; for a contrasting report in murine testis see, e.g., Hirai et al. 2012). Figure 8 shows differences of intensities between blood and lymph vessels. While small lymph vessels are very positive for endothelial markers, here claudin-5, they are mostly negative for SM markers (see, e.g., the SM α-actin reaction in Fig. 8a–a″). Another frequent view is that seen in the cross-sectioned vessels of Fig. 8b–b″. As both tight and adherens junctions (AJs) are located side-by-side, the limited light microscopic resolution often suggests colocalization, as also indicated by the small structures showing yellow merger color for claudin-5 and β-catenin (for similar observations, see also Baluk et al. 2007). By contrast, a far-reaching vessel wall versus endothelium reaction is seen for claudin-5 and desmin in Fig. 8c–c″.
Double-label immunofluorescence microscopy of cryostat cross-sections through STs of rat testis. Immunostaining reactions with antibodies to claudin-5 (a, a″, b, b″; red) and VE-cadherin (c, c″; red) show the exclusive occurrence of both marker molecules in vascular endothelia (arrows; V, vessel) and their complete absence in cells of the PW, shown by smooth muscle α-actin (α-SMA, a′, a″, b′, b″, c′, c″; green). L, lumen; I, Interstitial space. Bars 20 μm
Double-label immunofluorescence microscopy of cryostat cross-sections through STs of frozen rat testes after reactions of blood vessels (V) and lymph vessels (arrows) with antibodies to claudin-5 (a, a″, b, b″, c, c″; red) in comparison with antibodies against smooth muscle α-actin (α-SMA; a′, a″; green), β-catenin (b′, b″; green) and desmin (c′, c″; green). Note that the smaller lymph and blood vessel (V) endothelia are positive for the endothelial marker claudin-5 but negative for all three SMC markers. Note also the complete absence of an endothelial marker reaction in a cell layer covering the peritubular LSMC wall (4). L, lumen; I, interstitial space. Bars 50 μm
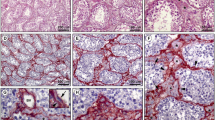
In principle, the immunostaining results are the same in the rodents examined as in the other mammalian species. Figure 9 shows, for example, in a guinea pig testis, relatively wide ST lumina with very narrow interstitial regions containing only rather small vascular structures that, however, are fully positive for the endothelial markers used. And in Fig. 10a–a‴, an extended section through a rather large part of a human testis is seen, showing a lymphatic vessel with an endothelium positive for both podoplanin (red) and LYVE-1 (green), here accompanied by some extremely small vascular structures positive for either one or the other.
Double-label immunofluorescence microscopy of a cryostat cross-section through STs of frozen guinea pig testis, showing immunostaining reactions of the specific endothelial tight junction marker claudin-5 (a, b, b″; red) and the SMC marker desmin (a, b′, b″; green). Note that the endothelia of both the—here rather small—lymphatic and blood (V) vessels are positive for the endothelial marker but negative for the SMC marker. Note again the complete absence of the endothelial marker reaction in the outermost cell layer covering the peritubular LSMC wall. L, lumen; I, interstitial space. Bars 50 μm (a), 20 μm (b″)
Double-label immunofluorescence microscopy of a cryostat section through STs of frozen human testis, showing positive immunostaining for vascular endothelium with both podoplanin (a, a″, a‴; red) and LYVE-1 (a′, a‴; green) in a relatively large vascular wall structure (V) whereas some very small vascular structures are only LYVE-1-positive (arrows). The arrowheads in a‴ denote a special kind of very small lymphatic endothelial structures, which here prominently react with podoplanin (red). Note the complete absence of both markers in the cells of the PW (brackets). As some sort of control the reaction of protein CD34 is shown in b–b‴ (red), in direct comparison with the LSMCs (green). The specific positive CD34 reaction on the PW surface cells demonstrates again that this protein is not endothelium-specific but occurs in a wide range of diverse mesenchymally and hematopoietically derived cells (for a review, see, e.g., Nielsen and McNagny 2008). L, lumen; I, interstitial space. Bar 50 μm
As already reported in previous publications, endothelial AJs are also markedly positive for protein myozap (e.g., Pieperhoff et al. 2012) as well as for proteins p120 and plakoglobin (for reviews, see Franke et al. 1987, 1988; Franke 2009; for endothelial structures and functions, see also, e.g., Iyer et al. 2004) and these proteins have also been found in the AJs of the LSMC monolayers of PWs (see, e.g., Domke and Franke 2019). Again, we have not discovered them in the outermost PW-associated layer. Finally, desmoplakin-positive complexus adhaerens structures as they occur in certain special endothelial tissues (e.g., Schmelz and Franke 1993; Schmelz et al. 1990, 1994; Valiron et al. 1996; Kowalczyk et al. 1998; Ebata et al. 2001; Hämmerling et al. 2006; Pfeiffer et al. 2008; Moll et al. 2009) have not been identified in any type of testicular blood or lymph vessel.
Special controls using antibodies against proteins CD34 and β-catenin
Immunofluorescence and immunoelectron microscopy for the detection of cytoskeletal and cell–cell junctional molecules in both endothelial as well as a wide range of mesenchymally derived cell types also gave the expected positive localization reactions in the endothelial cells of the blood and lymph vessels as described in previous publications (Franke et al. 1978, 1979, 1987, 1988; Pieperhoff et al. 2012). As an example, we show here the reaction of protein CD34 (for a relevant review, see Fiedler et al. 2006) in the outermost layer of the PWs but not in the SMCs (Fig. 10b–b‴). A similar but much weaker reaction was seen with antibodies to β-catenin.
Discussion
The clear conclusion of this study is that the very attenuated cells of the outermost layer associated with the PW of the STs of rodents and other mammalian species are not cells of a lymphatic endothelium and are not even endothelium-like cells in ultrastructural and molecular terms. As we have directly controlled our results by positive reactions of the endothelial cells located in the adjacent blood and lymph vessels in the interstitial space, the significance of our negative conclusion is obvious, for rodent testes as well as for all other mammalian species examined. The cells of this outermost PW-associated monolayer are also remarkable as they represent—to the best of our knowledge—in large parts the thinnest lamellar cells of the mammalian body in situ (11–25-nm inner cytoplasmic thickness), i.e., similar to the 9–11-nm inner cytoplasmic membrane-to-membrane thickness in the most attenuated cultured cells shown so far (Franke et al. 1978; for extended, very thin cell protrusions—often also with cell–cell junctions of the AJ type—in certain mammalian tissues see also Barth et al. 2009, 2012).
Consequently, it is also clear that for conclusions like this, the identification of cell type–specific major architectonic molecules by immunolocalization in situ is decisive, if based on reliably good antibodies. As such, immunolocalization-suitable antibodies have been developed only in the last few decades, earlier tissue and cell type nomenclature has been based on the appearance, position, or assumed function. The blood and lymph vessel system, for example, has interestingly been given function or position-oriented names, beginning with “sucking vessel” (“Saugader,” Hildebrandt 1802), “blood vessel” (“Blutader,” Virchow 1858) and finally “endothelium” (Arnold 1876) and “reticulo-endothelial system” (Aschoff 1924). In contrast, major architectonic, cell type–characteristic and thus diagnostically reliable molecules have been isolated, chemically examined and made detectable by specific antibodies as cytoskeletal or junctional elements only several decades later, i.e., far after 1980 (for relevant reviews on endothelial cells, see, e.g., Simionescu et al. 1982; Daróczy 1988; Gotlieb and Wong 1988; Jaffe 1988; Larson 1988; Palade 1988; Wagner 1988; Zetter 1988). Consequently, the basis for the identification and localization of stable and specific endothelial cells was only prepared in the 1990s, i.e., after the discovery of cell type–specific marker molecules such as VE-cadherin (Lampugnani et al. 1992, 1995; Navarro et al. 1998; Dejana 2004; Ferreri and Vincent 2008; Bravi et al. 2014), claudin-5 (Morita et al. 1999), protein LYVE-1 (Banerji et al. 1999; Prevo et al. 2001), podoplanin (Schacht et al. 2005; Cîmpean et al. 2007) and protein myozap (Seeger et al. 2010; Rickelt et al. 2011; Pieperhoff et al. 2012), which have laid the foundation for a systematic and reliable molecular diagnosis of lymphatic endothelial cells in situ (see, e.g., also Mäkinen et al. 2001; Sleeman et al. 2001; Podgrabinska et al. 2002; Baluk et al. 2007; Baluk and McDonald 2008; Noda et al. 2010).
On the other hand, the positive molecular classification and diagnosis of the cells of the outermost PW-covering monolayer (“fibrocytes” sensu Bressler and Ross 1972) has not yet been finished. As already mentioned, these cells are so extremely thin that special electron microscopy immunolocalization protocols had to be developed. We hope that these methods will soon allow an improved molecular biological identification of these cells.
Finally, we have to discuss some central problems of the previous articles of this series (Domke et al. 2014; Domke and Franke 2019) and the present one. In particular, we need to start a serious general discussion of publications in cell biology. The reason is that we had to learn that published incorrect results are not corrected by the responsible authors but just repeated again and again for several decades. In the field of male genital cell biology, for example, we had explicitly published the regular absence of desmosomes and desmosome-like structures as junctions connecting the Sertoli cells in the STs of mammalian testes (e.g., Franke et al. 1981, 1982, 1983; for recent reviews, see Franke 2009 and the Introduction in the report of Domke et al. 2014). Nevertheless, the list of these incorrect claims has been again repeated and even extended in recent years (see Introduction and Table S1 in Electronic Supplementary Material of Domke and Franke 2019). Now in the present report, we have cited some examples of incorrect results claiming the close and extended association of a lymphatic endothelium with the outermost SM cell layer of the PWs of the STs in several mammalian species, notably rodents, which as we have now extensively demonstrated is also incorrect. As the authors of such incorrect reports apparently do not correct their claims, the question arises how can one here protect the importance of the truth in science, in basic research as well as in physiology and medical research, including diagnosis and therapy.
Notes
VE-cadherin has also been noted—often only in local cell groups or colonies—in certain kinds of other cells (Boda-Heggemann et al. 2009).
Abbreviations
- AJ(s):
-
Adherens junction(s)
- ECM:
-
Extracellular matrix
- LSMC(s):
-
Lamellar smooth muscle cell(s)
- PW:
-
Peritubular wall
- SM:
-
Smooth muscle
- SMC(s):
-
Smooth muscle cell(s)
- ST(s):
-
Seminiferous tubule(s)
References
Arnold J (1876) [Ueber die Kittsubstanz der Endothelien.] Arch. Path. Anat. 64:77–108
Aschoff L (1924) Das reticulo-endotheliale System. In: Czerny A, Heubner O, Kraus F, Minkowski O, Müller F, Sahli H (eds) Ergebnisse der Inneren Medizin und Kinderheilkunde, vol 26. Springer-Verlag, Berlin, pp 1–118
Baluk P, McDonald DM (2008) Markers of micrAnn NY Acad Scioscopic imaging of lymphangiogenesis and angiogenesis. 1131, 1:–12
Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, McDonald DM (2007) Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 204:2349–2362
Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG (1999) LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol 144:789–801
Barth M, Schumacher H, Kuhn C, Akhyari P, Lichtenberg A, Franke WW (2009) Cordial connections: molecular ensembles and structures of adhering junctions connecting interstitial cells of cardiac valves in situ and in cell culture. Cell Tissue Res 337:63–77
Barth M, Rickelt S, Noffz E, Winter-Simanowski S, Niemann H, Akhyari P, Lichtenberg A, Franke WW (2012) The adhering junctions of valvular interstitial cells: molecular composition in fetal and adult hearts and the comings and goings of plakophilin-2 in situ, in cell culture and upon re-association with scaffolds. Cell Tissue Res 348:295–307
Boda-Heggemann J, Regnier-Vigouroux A, Franke WW (2009) Beyond vessels: occurrence and regional clustering of vascular endothelial (VE-)cadherin-containing junctions in non-endothelial cells. Cell Tissue Res 335:49–65
Bravi L, Dejana E, Lampugnani MG (2014) VE-cadherin at a glance. Cell Tissue Res 355:515–522
Bressler RS, Ross MH (1972) Differentiation of peritubular myoid cells of the testis: effects of intratesticular implantation of newborn mouse testes into normal and hypophysectomized adults. Biol Reprod 6:148–159
Bruyère F, Noël A (2010) Lymphangiogenesis: in vitro and in vivo models. FASEB J 24:8–21
Cîmpean AM, Raica M, Izvernariu DA, Tătucu D (2007) Lymphatic vessels identified with podoplanin. Comparison of immunostaining with three different detection systems. Rom J Morphol Embryol 48:139–143
Clark RV (1976) Three-dimensional organization of testicular interstitial tissue and lymphatic space in the rat. Anat Rec 184:203–225
Connell CJ (1976) A scanning electron microscope study of the interstitial tissue of the canine testis. Anat Rec 185:389–402
Cowin P, Franke WW, Grund C, Kapprell H-P, Kartenbeck J (1985a) The desmosome-intermediate filament complex. In: Edelman GM, Thiery J-P (eds) The cell in contact. John Wiley & Sons, New York, pp 427–460
Cowin P, Kapprell HP, Franke WW (1985b) The complement of desmosomal plaque proteins in different cell types. J Cell Biol 101:1442–1454
Cowin P, Kapprell HP, Franke WW, Tamkun J, Hynes RO (1986) Plakoglobin: a protein common to different kinds of intercellular adhering junctions. Cell 46:1063–1073
Daróczy J (1988) The dermal lymphatic capillaries. Springer, Berlin Heidelberg
Dejana E (2004) Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol 5:261–270
Dejana E, Lampugnani MG, Martinez-Estrada O, Bazzoni G (2000) The molecular organization of endothelial junctions and their functional role in vascular morphogenesis and permeability. Int J Dev Biol 44:743–748
Domke LM (2018) Molecular and ultrastructural characteristics of adhering junctions and cytoskeletons in cells of the seminiferous tubules and the peritubular walls of mammalian testes. PhD Thesis. Combined Faculties for the Natural Sciences and for Mathematics, University of Heidelberg, Germany
Domke LM, Franke WW (2019) The cell–cell junctions of mammalian testes: II. The smooth muscle monolayer cells of the peritubular wall are laterally connected in closely overlapping, lamelliform protrusions by vertical adherens junctions – a novel architectural cell junction system and a novel kind of muscle tissue. Cell Tissue Res 375:451–482
Domke LM, Rickelt S, Dörflinger Y, Kuhn C, Winter-Simanowski S, Zimbelmann R, Rosin-Arbesfeld R, Heid H, Franke WW (2014) The cell-cell junctions of mammalian testes: I. The adhering junctions of the seminiferous epithelium represent special differentiation structures. Cell Tissue Res 357:645–665
Dym M (1975) Male reproductive system. In: Bloom W, Fawcett DW (eds) A textbook of histology. Saunders Company, Philadelphia, pp 805–857
Dym M (1988) The male reproductive system. In: Weiss L (ed) Cell and tissue biology. A textbook of histology. Urban & Schwarzenberg, Baltimore, pp 929–972
Dym M (1994) Basement membrane regulation of Sertoli cells. Endocr Rev 15:102–115
Dym M, Fawcett DW (1970) The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod 3:308–326
Ebata N, Nodasaka Y, Sawa Y, Yamaoka Y, Makino S, Totsuka Y, Yoshida S (2001) Desmoplakin as a specific marker of lymphatic vessels. Microvasc Res 61:40–48
Fawcett DW, Heidger PM, Leak LV (1969) Lymph vascular system of the interstitial tissue of the testis as revealed by electron microscopy. J Reprod Fertil 19:109–119
Fawcett DW, Leak LV, Heidger PM Jr (1970) Electron microscopic observations on the structural components of the blood-testis barrier. J Reprod Fertil Suppl 10:105–122
Fawcett DW, Neaves WB, Flores MN (1973) Comparative observations on intertubular lymphatics and the organization of the interstitial tissue of the mammalian testis. Biol Reprod 9:500–532
Ferreri DM, Vincent PA (2008) Signaling to and through the endothelial adherens junction. In: Laflamme SE, Kowalczyk AP (eds) Cell junctions. Adhesion, development, and disease. Wiley-VCH, Weinheim, pp 169–195
Fiedler U, Christian S, Koidl S, Kerjaschki D, Emmett MS, Bates DO, Christofori G, Augustin HG (2006) The sialomucin CD34 is a marker of lymphatic endothelial cells in human tumors. Am J Pathol 168:1045–1053
Franke WW (2009) Discovering the molecular components of intercellular junctions – a historical view. Cold Spring Harb. Perspect. Biol. 1:a003061
Franke WW, Schmid E, Osborn M, Weber K (1978) Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc Natl Acad Sci U S A 75:5034–5038
Franke WW, Schmid E, Osborn M, Weber (1979) Intermediate-sized filaments of human endothelial cells. J Cell Biol 81:570–580
Franke WW, Schmid E, Grund C, Müller H, Engelbrecht I, Moll R, Stadler J, Jarasch ED (1981) Antibodies to high molecular weight polypeptides of desmosomes: specific localization of a class of junctional proteins in cells and tissue. Differentiation 20:217–241
Franke WW, Moll R, Schiller DL, Schmid E, Kartenbeck J, Mueller H (1982) Desmoplakins of epithelial and myocardial desmosomes are immunologically and biochemically related. Differentiation 23:115–127
Franke WW, Moll R, Mueller H, Schmid E, Kuhn C, Krepler R, Artlieb U, Denk H (1983) Immunocytochemical identification of epithelium-derived human tumors with antibodies to desmosomal plaque proteins. Proc Natl Acad Sci USA 80:543–547
Franke WW, Kapprell HP, Cowin P (1987) Immunolocalization of plakoglobin in endothelial junctions: identification as a special type of Zonulae adhaerentes. Biol Cell 59:205–218
Franke WW, Cowin P, Grund C, Kuhn C, Kapprell H-P (1988) The endothelial junction. The plaque and its components. In: Simionescu N, Simionescu M (eds) Endothelial cell biology in health and disease. Plenum Press, New York, pp 147–166
Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S (1993) Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol 123:1777–1788
Giannotta M, Trani M, Dejana E (2013) VE-Cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev Cell 26:441–454
Gotlieb AI, Wong MKK (1988) Current concepts on the role of the endothelial cytoskeleton in endothelial integrity, repair, and dysfunction. In: Ryan US (ed) Endothelial cells. Vol II. CRC Press, Boca Raton, pp 81–101
Hadley MA, Dym M (1987) Immunocytochemistry of extracellular matrix in the lamina propria of the rat testis: electron microscopic localization. Biol Reprod 37:1283–1289
Hämmerling B, Grund C, Boda-Heggemann J, Moll R, Franke WW (2006) The complexus adhaerens of mammalian lymphatic endothelia revisited: a junction even more complex than hitherto thought. Cell Tissue Res 324:55–67
Hildebrandt F (1802) Von den Saugadern überhaupt. In: Lehrbuch der Anatomie des Menschen. Vol 4. Ghelen, Wien, pp 189–200
Hirai S, Naito M, Terayama H, Qu N, Kuerban M, Musha M, Ikeda A, Miura M, Itoh M (2012) The origin of lymphatic capillaries in murine testes. J Androl 33:745–751
Hofmann I, Kuhn C, Franke WW (2008) Protein p0071, a major plaque protein of non-desmosomal adhering junctions, is a selective cell-type marker. Cell Tissue Res 334:381–399
Hofmann I, Schlechter T, Kuhn C, Hergt M, Franke WW (2009) Protein p0071 - an armadillo plaque protein that characterizes a specific subtype of adherens junctions. J Cell Sci 122:21–24
Holstein AF, Orlandini GE, Möller R (1979) Distribution and fine structure of the lymphatic system in the human testis. Cell Tissue Res 200:15–27
Iyer S, Ferreri DM, DeCocco NC, Minnear FL, Vincent PA (2004) VE-cadherin-p120 interaction is required for maintenance of endothelial barrier function. Am J Physiol Lung Cell Mol Physiol 286:L1143–L1153
Jaffe EA (1988) Synthesis of von Willebrand Factor by endothelial cells. In: Ryan US (ed) Endothelial cells. Vol I. CRC Press, Boca Raton, pp 119–126
Ji RC, Eshita Y, Kato S (2007) Investigation of intratumoural and peritumoural lymphatics expressed by podoplanin and LYVE-1 in the hybridoma-induced tumours. Int J Exp Pathol 88:257–270
Jussila L, Alitalo K (2002) Vascular growth factors and lymphangiogenesis. Physiol Rev 82:673–700
Karpanen T, Alitalo K (2008) Molecular biology and pathology of lymphangiogenesis. Annu Rev Pathol Mech Dis 3:367–397
Koeser J, Troyanovsky SM, Grund C, Franke WW (2003) De novo formation of desmosomes in cultured cells upon transfection of genes encoding specific desmosomal components. Exp Cell Res 285:114–130
Kowalczyk AP, Navarro P, Dejana E, Bornslaeger EA, Green KJ, Kopp DS, Borgwardt JE (1998) VE-cadherin and desmoplakin are assembled into dermal microvascular endothelial intercellular junctions: a pivotal role for plakoglobin in the recruitment of desmoplakin to intercellular junctions. J Cell Sci 111:3045–3057
Lampugnani MG, Resnati M, Raiteri M, Pigott R, Pisacane A, Houen G, Ruco LP, Dejana E (1992) A novel endothelial-specific membrane protein is a marker of cell-cell contacts. J Cell Biol 118:1511–1522
Lampugnani MG, Corada M, Caveda L, Breviario F, Ayalon O, Geiger B, Dejana E (1995) The molecular organization of endothelial cell to cell junctions: differential association of plakoglobin, beta-catenin, and alpha-catenin with vascular endothelial cadherin (VE-cadherin). J Cell Biol 129:203–217
Larson DM (1988) Intercellular junctions and junctional transfer in the blood vessel wall. In: Ryan US (ed) Endothelial cells. Vol III. CRC Press, Boca Raton, pp 75–88
Leak LV (1970) Electron microscopic observations on lymphatic capillaries and the structural components of the connective tissue-lymph interface. Microvasc Res 2:361–391
Leak LV (1971) Studies on the permeability of lymphatic capillaries. J Cell Biol 50:300–323
Leak LV (1976) The structure of lymphatic capillaries on lymph formation. Federation Proc 35:1863–1871
Leak LV, Burke JF (1968) Ultrastructural studies on the lymphatic anchoring filaments. J Cell Biol 36:129–149
Losinno AD, Morales A, Fernandez D, Lopez LA (2012) Peritubular myoid cells from rat seminiferous tubules contain actin and myosin filaments distributed in two independent layers. Biol Reprod 86(150):151–158
Losinno AD, Sorrivas V, Ezquer M, Ezquer F, Lopez LA, Morales A (2016) Changes of myoid and endothelial cells in the peritubular wall during contraction of the seminiferous tubule. Cell Tissue Res 365:425–435
Luttun A, Tjwa M, Carmeliet P (2004) Formation of blood and lymphatic vessels: role of progenitors. In: Lanza R, Blau H, Melton D, Moore M, Thomas ED, Verfaillie C, Weissman I, West M (eds) Handbook of stem cells, vol 2. Adult and fetal stem cells. Elsevier Academic Press, Amsterdam, pp 455–474
Maekawa M, Kamimura K, Nagano T (1996) Peritubular myoid cells in the testis: their structure and function. Arch Histol Cytol 59:1–13
Mäkinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, Wise L, Mercer A, Kowalski H, Kerjaschki D, Stacker SA, Achen MG, Alitalo K (2001) Isolated, lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J 20:4762–4773
Mertens C, Kuhn C, Franke WW (1996) Plakophilins 2a and 2b: constitutive proteins of dual location in the karyoplasm and the desmosomal plaque. J Cell Biol 135:1009–1025
Moll R, Sievers E, Hämmerling B, Schmidt A, Barth M, Kuhn C, Grund C, Hofmann I, Franke WW (2009) Endothelial and virgultar cell formations in the mammalian lymph node sinus: endothelial differentiation morphotypes characterized by a special kind of junction (complexus adhaerens). Cell Tissue Res 335:109–141
Morita K, Sasaki H, Furuse M, Tsukita S (1999) Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol 147:185–194
Moroi S, Saitou M, Fujimoto K, Sakakibara A, Furuse M, Yoshida O, Tsukita S (1998) Occludin is concentrated at tight junctions of mouse/rat but not human/guinea pig Sertoli cells in testes. Am J Physiol 274:C1708–C1717
Navarro P, Ruco, Dejana E (1998) Differential localization of VE- and N-cadherins in human endothelial cells: VE-cadherin competes with N-cadherin for junctional localization. J Cell Biol 140:1475–1484
Nielsen JS, McNagny KM (2008) Novel functions of the CD34 family. J Cell Sci 121:3683–3692
Noda Y, Amano I, Hata M, Kojima H, Sawa Y (2010) Immunohistochemical examination on the distribution of cells expressed lymphatic endothelial marker podoplanin and LYVE-1 in the mouse tongue tissue. Acta Histochem Cytochem 43:61–68
Nose A, Takeichi M (1986) A novel cadherin cell adhesion molecule: its expression patterns associated with implantation and organogenesis of mouse embryos. J Cell Biol 103:2649–2658
Ozawa M, Ringwald M, Kemler R (1990) Uvomorulin-catenin complex formation is regulated by a specific domain in the cytoplasmic region of the cell adhesion molecule. Proc Natl Acad Sci U S A 87:4246–4250
Palade GE (1988) The microvascular endothelium revisited. In: Simionescu N, Simionescu M (eds) Endothelial cell biology in health and disease. Plenum Press, New York London, pp 3–22
Pfeiffer F, Kumar V, Butz S, Vestweber D, Imhof BA, Stein JV, Engelhardt B (2008) Distinct molecular composition of blood and lymphatic vascular endothelial cell junctions establishes specific functional barriers within the peripheral lymph node. Eur J Immunol 38:2142–2155
Pieperhoff S, Rickelt S, Heid H, Claycomb WC, Zimbelmann R, Kuhn C, Winter-Simanowski S, Frey N, Franke WW (2012) The plaque protein myozap identified as a novel major component of adhering junctions in endothelia of the blood and the lymph vascular systems. J Cell Mol Med 16:1709–1719
Podgrabinska S, Braun P, Velasco P, Kloos B, Pepper MS, Skobe M (2002) Molecular characterization of lymphatic endothelial cells. Proc Natl Acad Sci U S A 99:16069–16074
Prevo R, Banerji S, Ferguson DJ, Clasper S, Jackson DG (2001) Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J Biol Chem 276:19420–19430
Reynolds AB, Daniel J, McCrea PD, Wheelock MJ, Wu J, Zhang Z (1994) Identification of a new catenin: the tyrosine kinase substrate p120cas associates with E-cadherin complexes. Mol Cell Biol 14:8333–8342
Reynolds AB, Daniel JM, Mo YY, Wu J, Zhang Z (1996) The novel catenin p120cas binds classical cadherins and induces an unusual morphological phenotype in NIH3T3 fibroblasts. Exp Cell Res 225:328–337
Rickelt S, Rizzo S, Dörflinger Y, Zentgraf H, Basso C, Gerosa G, Thiene G, Moll R, Franke WW (2010) A novel kind of tumor type-characteristic junction: plakophilin-2 as a major protein of adherens junctions in cardiac myxomata. Mod Pathol 23:1429–1437
Rickelt S, Kuhn C, Winter-Simanowski S, Zimbelmann R, Frey N, Franke WW (2011) Protein myozap – a late addition to the molecular ensembles of various kinds of adherens junctions. Cell Tissue Res 346:347–359
Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Inazawa J, Fujimoto K, Tsukita S (1997) Mammalian occludin in epithelial cells: its expression and subcellular distribution. Eur J Cell Biol 73:222–231
Schacht V, Dadras SS, Johnson LA, Jackson DG, Hong Y-K, Detmar M (2005) Up-regulation of the lymphatic marker podoplanin, a mucin-type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am J Pathol 166:913–921
Schäfer S, Koch PJ, Franke WW (1994) Identification of the ubiquitous human desmoglein, Dsg2, and the expression catalogue of the desmoglein subfamily of desmosomal cadherins. Exp Cell Res 211:391–399
Schäfer S, Stumpp S, Franke WW (1996) Immunological identification and characterization of the desmosomal cadherin Dsg2 in coupled and uncoupled epithelial cells and in human tissues. Differentiation 60:99–108
Schmelz M, Franke WW (1993) Complexus adhaerentes, a new group of desmoplakin-containing junctions in endothelial cells: the syndesmos connecting retothelial cells of lymph nodes. Eur J Cell Biol 61:274–289
Schmelz M, Moll R, Franke WW (1990) A new type of intercellular junction: desmosomal proteins in the extended junctions of certain endothelial cells of the lymphatic systems. Cell Biol Int Rep 14:54
Schmelz M, Moll R, Kuhn C, Franke WW (1994) Complexus adhaerentes, a new group of desmoplakin-containing junctions in endothelial cells: II. Different types of lymphatic vessels. Differentiation 57:97–117
Seeger TS, Frank D, Rohr C, Will R, Just S, Grund C, Lyon R, Luedde M, Koegl M, Sheikh F, Rottbauer W, Franke WW, Katus HA, Olson EN, Frey N (2010) Myozap, a novel intercalated disc protein, activates serum response factor-dependent signaling and is required to maintain cardiac function in vivo. Circ Res 106:880–890
Simionescu M, Simionescu N, Palade GE (1982) Biochemically differentiated microdomains of the cell surface of capillary endothelium. Ann NY Acad Sci 401:9–24
Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G (1986) A monoclonal antibody against α-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol 103:2787–2796
Sleeman JP, Krishnan J, Kirkin V, Baumann P (2001) Markers for the lymphatic endothelium: in search of the holy grail? Microsc Res Tech 55:61–69
Söderström KO (1981) Scanning electron microscopy of mechanically isolated seminiferous tubules of the rat testis. Andrologia 13:155–161
Suzuki K, Fukusumi Y, Yamazaki M, Kaneko H, Tsuruga K, Tanaka H, Ito E, Matsui K, Kawachi H (2015) Alteration in the podoplanin-ezrin-cytoskeleton linkage is an important initiation event of the podocyte injury in puromycin aminonucleoside nephropathy, a mimic of minimal change nephrotic syndrome. Cell Tissue Res 362:201–213
Takeichi M (1990) Cadherins: a molecular family important in selective cell-cell adhesion. Annu Rev Biochem 59:237–252
Valiron O, Chevrier V, Usson Y, Breviario F, Job D, Dejana E (1996) Desmoplakin expression and organization at human umbilical vein endothelial cell-to-cell junctions. J Cell Sci 109:2141–2149
van Muijen GN, Ruiter DJ, Warnaar SO (1987) Coexpression of intermediate filament polypeptides in human fetal and adult tissues. Lab Invest 57:359–369
Virchow R (1858) Die Cellularpathologie in ihrer Begründung auf physiologische und pathologische Gewebelehre. Verlag von August Hirschwald, Berlin
Vorbrodt AW, Dobrogowska DH (2004) Molecular anatomy of interendothelial junctions in human blood-brain barrier microvessels. Folia Histochem Cytobiol 42:67–75
Wagner RC (1988) Ultrastructural studies of capillary endothelium. Compartmental tracing, high-voltage electron microscopy, and cryofixation. In: Simionescu N, Simionescu M (eds) Endothelial cell biology in health and disease. Plenum Press, New York London, pp 23–47
Weiss L (1983) Lymphatic vessels and lymph nodes. In: Weiss L (ed) Cell and tissue biology. A textbook of histology. Urban & Schwarzenberg, Baltimore, pp 497–514
Wrobel KH, Mademann R, Sinowatz F (1979) The lamina propria of the bovine seminiferous tubule. Cell Tissue Res 202:357–377
Wrobel KH, Sinowatz F, Mademann R (1981) Intertubular topography in the bovine testis. Cell Tissue Res 217:289–310
Yazama F, Esaki M, Sawada H (1997) Immunocytochemistry of extracellular matrix components in the rat seminiferous tubule: electron microscopic localization with improved methodology. Anat Rec 248:51–62
Zetter BR (1988) Endothelial heterogeneity: influence of vessel size, organ localization, and species specificity on the properties of cultured endothelial cells. In: Ryan US (ed) Endothelial cells. Vol II. CRC Press, Boca Raton, pp 63–79
Funding
Funding of this study by the authors’ home institution, the German Cancer Research Center, DKFZ, Heidelberg, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national and institutional guidelines for the care and use of animals were followed in the procurement of testicular tissue samples from rats and mice (source: Central Animal Laboratory of the German Cancer Research Center, Heidelberg, Germany), from bulls (source: local slaughterhouse in Mannheim, Germany) and from boars (source: Institute of Farm Animal Genetics, Friedrich-Loeffler-Institute, Mariensee, Germany). Cryopreserved and aldehyde-fixed human testis samples were obtained from surgical material taken, examined for diagnostic pathology and processed in compliance with the regulations of the Ethics Committee of the University of Heidelberg, Germany, in accordance with the ethical standards of the Federal Research Committee of Germany and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is written in respect of the cell biology work of Donald W. Fawcett, who certainly would have been the first to correct his former conclusions based only on the electron microscopical appearance of the testicular histology of rodents.
Electronic supplementary material
ESM 1
(PDF 7.94 mb).
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Franke, W.W., Domke, L.M., Dörflinger, Y. et al. The cell–cell junctions of mammalian testes. III. Absence of an endothelial cell layer covering the peritubular wall of the seminiferous tubules—an immunocytochemical correction of a 50-year-old error in the literature. Cell Tissue Res 379, 75–92 (2020). https://doi.org/10.1007/s00441-019-03116-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-019-03116-5