Abstract
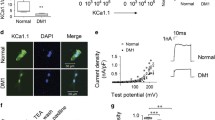
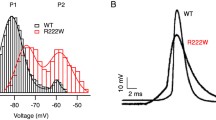
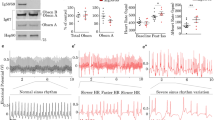
Andersen’s syndrome (AS) is a rare autosomal disorder that has been defined by the triad of periodic paralysis, cardiac arrhythmia, and developmental anomalies. AS has been directly linked to over 40 different autosomal dominant negative loss-of-function mutations in the KCNJ2 gene, encoding for the tetrameric strong inward rectifying K+ channel KIR2.1. While KIR2.1 channels have been suggested to contribute to setting the resting membrane potential (RMP) and to control the duration of the action potential (AP) in skeletal and cardiac muscle, the mechanism by which AS mutations produce such complex pathophysiological symptoms is poorly understood. Thus, we use an adenoviral transduction strategy to study in vivo subcellular distribution of wild-type (WT) and AS-associated mutant KIR2.1 channels in mouse skeletal muscle. We determined that WT and D71V AS mutant KIR2.1 channels are localized to the sarcolemma and the transverse tubules (T-tubules) of skeletal muscle fibers, while the ∆314-315 AS KIR2.1 mutation prevents proper trafficking of the homo- or hetero-meric channel complexes. Whole-cell voltage-clamp recordings in individual skeletal muscle fibers confirmed the reduction of inwardly rectifying K+ current (IK1) after transduction with ∆314-315 KIR2.1 as compared to WT channels. Analysis of skeletal muscle function revealed reduced force generation during isometric contraction as well as reduced resistance to muscle fatigue in extensor digitorum longus muscles transduced with AS mutant KIR2.1. Together, these results suggest that KIR2.1 channels may be involved in the excitation–contraction coupling process required for proper skeletal muscle function. Our findings provide clues to mechanisms associated with periodic paralysis in AS.








Similar content being viewed by others
References
Adrian RH, Bryant SH (1974) On the repetitive discharge in myotonic muscle fibres. J Physiol 240:505–515
Adrian RH, Chandler WK, Hodgkin AL (1970) Voltage clamp experiments in striated muscle fibres. J Physiol 208:607–644
Adrian RH, Marshall MW (1976) Action potentials reconstructed in normal and myotonic muscle fibres. J Physiol 258:125–143
Allen DG, Lamb GD, Westerblad H (2008a) Impaired calcium release during fatigue. J Appl Physiol 104:296-305
Allen DG, Lamb GD, Westerblad H (2008b) Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88:287-332
Allen DG, Westerblad H (2001) Role of phosphate and calcium stores in muscle fatigue. J Physiol 536:657–665
Allen DG, Westerblad H, Lee JA, Lannergren J (1992) Role of excitation-contraction coupling in muscle fatigue. Sports Med 13:116-126
Almers W (1972) Potassium conductance changes in skeletal muscle and the potassium concentration in the transverse tubules. J Physiol 225:33–56
Andersen ED, Krasilnikoff PA, Overvad H (1971) Intermittent muscular weakness, extrasystoles, and multiple developmental anomalies. A new syndrome? Acta Paediatr Scand 60:559–564
Ashcroft FM, Heiny JA, Vergara J (1985) Inward rectification in the transverse tubular system of frog skeletal muscle studied with potentiometric dyes. J Physiol 359:269–291
Ashen MD, O'Rourke B, Kluge KA, Johns DC, Tomaselli GF (1995) Inward rectifier K+ channel from human heart and brain: cloning and stable expression in a human cell line. Am J Phys 268:H506–H511
Ballester LY, Benson DW, Wong B, Law IH, Mathews KD, Vanoye CG, George AL Jr (2006) Trafficking-competent and trafficking-defective KCNJ2 mutations in Andersen syndrome. Hum Mutat 27:388
Ballester LY, Vanoye CG, George AL, Jr. (2007) Exaggerated Mg2+ inhibition of Kir2.1 As a consequence of reduced PIP2 sensitivity in Andersen syndrome. Channels (Austin) 1:209-217
Bendahhou S, Donaldson MR, Plaster NM, Tristani-Firouzi M, Fu YH, Ptacek LJ (2003) Defective potassium channel Kir2.1 Trafficking underlies Andersen-Tawil syndrome. J Biol Chem 278:51779–51785
Chew TL, Wolf WA, Gallagher PJ, Matsumura F, Chisholm RL (2002) A fluorescent resonant energy transfer-based biosensor reveals transient and regional myosin light chain kinase activation in lamella and cleavage furrows. J Cell Biol 156:543–553
Christe G (1999) Localization of K(+) channels in the tubules of cardiomyocytes as suggested by the parallel decay of membrane capacitance, IK(1) and IK(ATP) during culture and by delayed IK(1) response to barium. J Mol Cell Cardiol 31:2207–2213
Citi S, Kendrick-Jones J (1987) Regulation of non-muscle myosin structure and function. BioEssays 7:155–159
Clark RB, Tremblay A, Melnyk P, Allen BG, Giles WR, Fiset C (2001) T-tubule localization of the inward-rectifier K(+) channel in mouse ventricular myocytes: a role in K(+) accumulation. J Physiol 537:979–992
Collet C, Pouvreau S, Csernoch L, Allard B, Jacquemond V (2004) Calcium signaling in isolated skeletal muscle fibers investigated under "silicone voltage-clamp" conditions. Cell Biochem Biophys 40:225–236
DiFranco M, Yu C, Quinonez M, Vergara JL (2015) Inward rectifier potassium currents in mammalian skeletal muscle fibres. J Physiol 593:1213–1238
Donaldson MR, Jensen JL, Tristani-Firouzi M, Tawil R, Bendahhou S, Suarez WA, Cobo AM, Poza JJ, Behr E, Wagstaff J, Szepetowski P, Pereira S, Mozaffar T, Escolar DM, Fu YH, Ptacek LJ (2003) PIP2 Binding residues of Kir2.1 Are common targets of mutations causing Andersen syndrome. Neurology 60:1811–1816
Doupnik CA, Davidson N, Lester HA (1995) The inward rectifier potassium channel family. Curr Opin Neurobiol 5:268–277
Dulhunty AF, Haarmann CS, Green D, Laver DR, Board PG, Casarotto MG (2002) Interactions between dihydropyridine receptors and ryanodine receptors in striated muscle. Prog Biophys Mol Biol 79:45–75
Endo M (1972) Stretch-induced increase in activation of skinned muscle fibres by calcium. Nat New Biol 237:211–213
Fitts RH (1994) Cellular mechanisms of muscle fatigue. Physiol Rev 74:49–94
Fontaine B, Vale-Santos J, Jurkat-Rott K, Reboul J, Plassart E, Rime CS, Elbaz A, Heine R, Guimaraes J, Weissenbach J et al (1994) Mapping of the hypokalaemic periodic paralysis (HypoPP) locus to chromosome 1q31-32 in three European families. Nat Genet 6:267–272
Graham FL, Rudy J, Brinkley P (1989) Infectious circular DNA of human adenovirus type 5: regeneration of viral DNA termini from molecules lacking terminal sequences. EMBO J 8:2077–2085
Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391:85–100
Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y (2010) Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev 90:291–366
Inagaki N, Tsuura Y, Namba N, Masuda K, Gonoi T, Horie M, Seino Y, Mizuta M, Seino S (1995) Cloning and functional characterization of a novel ATP-sensitive potassium channel ubiquitously expressed in rat tissues, including pancreatic islets, pituitary, skeletal muscle, and heart. J Biol Chem 270:5691–5694
Kokunai Y, Nakata T, Furuta M, Sakata S, Kimura H, Aiba T, Yoshinaga M, Osaki Y, Nakamori M, Itoh H, Sato T, Kubota T, Kadota K, Shindo K, Mochizuki H, Shimizu W, Horie M, Okamura Y, Ohno K, Takahashi MP (2014) A Kir3.4 Mutation causes Andersen-Tawil syndrome by an inhibitory effect on Kir2.1. Neurology 82:1058–1064
Kristensen M, Hansen T, Juel C (2006) Membrane proteins involved in potassium shifts during muscle activity and fatigue. Am J Physiol 290:R766–R772
Lesage F, Fink M, Barhanin J, Lazdunski M, Mattei MG (1995) Assignment of human G-protein-coupled inward rectifier K+ channel homolog GIRK3 gene to chromosome 1q21-q23. Genomics 29:808–809
Lim BC, Kim GB, Bae EJ, Noh CI, Hwang H, Kim KJ, Hwang YS, Ko TS, Chae JH (2010) Andersen cardiodysrhythmic periodic paralysis with KCNJ2 mutations: a novel mutation in the pore selectivity filter residue. J Child Neurol 25:490–493
Lopatin AN, Nichols CG (2001) Inward rectifiers in the heart: an update on I(K1). J Mol Cell Cardiol 33:625–638
Ma D, Taneja TK, Hagen BM, Kim BY, Ortega B, Lederer WJ, Welling PA (2011) Golgi export of the Kir2.1 Channel is driven by a trafficking signal located within its tertiary structure. Cell 145:1102–1115
Miake J, Marban E, Nuss HB (2003) Functional role of inward rectifier current in heart probed by Kir2.1 Overexpression and dominant-negative suppression. J Clin Invest 111:1529–1536
Movsesian MA, Schwinger RH (1998) Calcium sequestration by the sarcoplasmic reticulum in heart failure. Cardiovasc Res 37:352–359
Plaster NM, Tawil R, Tristani-Firouzi M, Canun S, Bendahhou S, Tsunoda A, Donaldson MR, Iannaccone ST, Brunt E, Barohn R, Clark J, Deymeer F, George AL Jr, Fish FA, Hahn A, Nitu A, Ozdemir C, Serdaroglu P, Subramony SH, Wolfe G, Fu YH, Ptacek LJ (2001) Mutations in Kir2.1 Cause the developmental and episodic electrical phenotypes of Andersen's syndrome. Cell 105:511–519
Priori SG, Pandit SV, Rivolta I, Berenfeld O, Ronchetti E, Dhamoon A, Napolitano C, Anumonwo J, di Barletta MR, Gudapakkam S, Bosi G, Stramba-Badiale M, Jalife J (2005) A novel form of short QT syndrome (SQT3) is caused by a mutation in the KCNJ2 gene. Circ Res 96:800–807
Raab-Graham KF, Radeke CM, Vandenberg CA (1994) Molecular cloning and expression of a human heart inward rectifier potassium channel. Neuroreport 5:2501–2505
Reiken S, Lacampagne A, Zhou H, Kherani A, Lehnart SE, Ward C, Huang F, Gaburjakova M, Gaburjakova J, Rosemblit N, Warren MS, He KL, Yi GH, Wang J, Burkhoff D, Vassort G, Marks AR (2003) PKA phosphorylation activates the calcium release channel (ryanodine receptor) in skeletal muscle: defective regulation in heart failure. J Cell Biol 160:919–928
Ryan DP, da Silva MR, Soong TW, Fontaine B, Donaldson MR, Kung AW, Jongjaroenprasert W, Liang MC, Khoo DH, Cheah JS, Ho SC, Bernstein HS, Maciel RM, Brown RH Jr, Ptacek LJ (2010) Mutations in potassium channel Kir2.6 Cause susceptibility to thyrotoxic hypokalemic periodic paralysis. Cell 140:88–98
Sacco S, Giuliano S, Sacconi S, Desnuelle C, Barhanin J, Amri EZ, Bendahhou S (2014) The inward rectifier potassium channel Kir2.1 is required for osteoblastogenesis. Hum Mol Genet 24:471–479
Sacconi S, Simkin D, Arrighi N, Chapon F, Larroque MM, Vicart S, Sternberg D, Fontaine B, Barhanin J, Desnuelle C, Bendahhou S (2009) Mechanisms underlying Andersen's syndrome pathology in skeletal muscle are revealed in human myotubes. Am J Physiol 297:C876–C885
Sakura H, Ammala C, Smith PA, Gribble FM, Ashcroft FM (1995a) Cloning and functional expression of the cDNA encoding a novel ATP-sensitive potassium channel subunit expressed in pancreatic beta-cells, brain, heart and skeletal muscle. FEBS Lett 377:338-344
Sakura H, Bond C, Warren-Perry M, Horsley S, Kearney L, Tucker S, Adelman J, Turner R, Ashcroft FM (1995b) Characterization and variation of a human inwardly-rectifying-K-channel gene (KCNJ6): a putative ATP-sensitive K-channel subunit. FEBS Lett 367:193-197
Sejersted OM, Sjogaard G (2000) Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol Rev 80:1411–1481
Standen NB, Stanfield PR (1978) A potential- and time-dependent blockade of inward rectification in frog skeletal muscle fibres by barium and strontium ions. J Physiol 280:169–191
Stoffel M, Espinosa R 3rd, Powell KL, Philipson LH, Le Beau MM, Bell GI (1994) Human G-protein-coupled inwardly rectifying potassium channel (GIRK1) gene (KCNJ3): localization to chromosome 2 and identification of a simple tandem repeat polymorphism. Genomics 21:254–256
Tan SV, Z'Graggen WJ, Boerio D, Rayan DL, Howard R, Hanna MG, Bostock H (2012) Membrane dysfunction in Andersen-Tawil syndrome assessed by velocity recovery cycles. Muscle Nerve 46:193–203
Tristani-Firouzi M, Jensen JL, Donaldson MR, Sansone V, Meola G, Hahn A, Bendahhou S, Kwiecinski H, Fidzianska A, Plaster N, Fu YH, Ptacek LJ, Tawil R (2002) Functional and clinical characterization of KCNJ2 mutations associated with LQT7 (Andersen syndrome). J Clin Invest 110:381–388
Tucker SJ, James MR, Adelman JP (1995) Assignment of KATP-1, the cardiac ATP-sensitive potassium channel gene (KCNJ5), to human chromosome 11q24. Genomics 28:127–128
Vaidyanathan R, Taffet SM, Vikstrom KL, Anumonwo JM (2010) Regulation of cardiac inward rectifier potassium current (I(K1)) by synapse-associated protein-97. J Biol Chem 285:28000–28009
Ward CW, Reiken S, Marks AR, Marty I, Vassort G, Lacampagne A (2003) Defects in ryanodine receptor calcium release in skeletal muscle from post-myocardial infarct rats. FASEB J 17:1517–1519
Yamada T, Mishima T, Sakamoto M, Sugiyama M, Matsunaga S, Wada M (2006) Oxidation of myosin heavy chain and reduction in force production in hyperthyroid rat soleus. J Appl Physiol (1985) 100:1520–1526
Yang J, Jan YN, Jan LY (1995) Determination of the subunit stoichiometry of an inwardly rectifying potassium channel. Neuron 15:1441–1447
Yano H, Philipson LH, Kugler JL, Tokuyama Y, Davis EM, Le Beau MM, Nelson DJ, Bell GI, Takeda J (1994) Alternative splicing of human inwardly rectifying K+ channel ROMK1 mRNA. Mol Pharmacol 45:854–860
Yoon G, Quitania L, Kramer JH, Fu YH, Miller BL, Ptacek LJ (2006) Andersen-Tawil syndrome: definition of a neurocognitive phenotype. Neurology 66:1703–1710
Acknowledgments
This work was supported by the Centre National de la Recherche Scientifique (CNRS) and Association Française contre les Myopathies (AFM) grant (S.B.) and Chateaubriand fellowships (D.S.) and City of Nice fellowship (S.G. and A.V.). We thank the Vector Core of the University Hospital of Nantes for providing the adenovirus vectors.
Conflict of interest
The authors declare having no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Simkin, D., Robin, G., Giuliano, S. et al. Andersen’s syndrome mutants produce a knockdown of inwardly rectifying K+ channel in mouse skeletal muscle in vivo. Cell Tissue Res 371, 309–323 (2018). https://doi.org/10.1007/s00441-017-2696-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-017-2696-7