Abstract
For over 100 years, scientists have tried to understand the mechanisms that lead to the axonal growth seen during development or the lack thereof during regeneration failure after spinal cord injury (SCI). Deoxyribozyme technology as a potential therapeutic to treat SCIs or other insults to the brain, combined with a bioinformatics approach to comprehend the complex protein-protein interactions that occur after such trauma, is the focus of this review. The reader will be provided with information on the selection process of deoxyribozymes and their catalytic sequences, on the mechanism of target digestion, on modifications, on cellular uptake and on therapeutic applications and deoxyribozymes are compared with ribozymes, siRNAs and antisense technology. This gives the reader the necessary knowledge to decide which technology is adequate for the problem at hand and to design a relevant agent. Bioinformatics helps to identify not only key players in the complex processes that occur after SCI but also novel or less-well investigated molecules against which new knockdown agents can be generated. These two tools used synergistically should facilitate the pursuit of a treatment for insults to the central nervous system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The first true-to-detail drawings of struggling axons at injury sites in the central or peripheral nerves system (CNS or PNS) were prepared from 1887 to 1903 by Santiago Ramon y Cajal (1852–1934). Since then, a plethora of publications have been generated in an attempt to comprehend the events that occur after CNS or PNS injury. Over time, a large number of genes, proteins and substances have been discovered and collected, all of which are involved in and are able to influence these processes. Although considerable improvements can be observed with regard to most of the promising candidates, satisfying results in treating insults to the CNS have not been achieved. Early responses to prevent complications and accelerate recovery are necessary during the medication of CNS trauma. Hence, the availability of safe and easy to administer therapeutics is long-awaited and the pursuit of a treatment or even a cure for paralysis continues.
Knockdown agents such as deoxyribozymes might be relevant in this regard. They have the ability to act as enzymes after binding to their target mRNA via sequence-specific binding-arms and cleaving the target via a catalytic loop structure. After releasing the digested fragments, the deoxyribozyme is able to bind and cleave further mRNA molecules of the same gene. Deoxyribozymes have been overlooked during the last few years but harbor great therapeutic potential. This review summarizes the selection process of deoxyribozyme development, the digestion mechanism, requirements for modifications of the length of the sequence-specific binding arms, existing knowledge about the uptake mechanism of nucleic-acid-based molecules into cells and therapeutic applications. Furthermore, to comprehend the multifactotrial and complex mechanisms that occur after CNS trauma, the review also summarizes results obtained via bioinformatics approaches.
Deoxyribozymes
Selection of deoxyribozyme sequences
Deoxyribozymes are single-stranded DNA molecules, which are also called DNA enzymes or DNAzyme. They were developed by Breaker and Joyce (1994) and Santoro and Joyce (1997) by using systematic evolution of ligands by exponential enrichment (SELEX; Fig. 1). The idea of developing catalytic DNA molecules arose because RNA in the form of ribozymes is able to function as an enzyme. However, the simple translation of a ribozyme sequence into DNA (replacing U with T) did not show satisfying results. No detectable cleavage of the target mRNA occurred. As a consequence, a random search for DNA sequences was undertaken by using SELEX. The first attempt resulted in Pb2+-dependent deoxyribozymes, one being called E2 (Fig. 2a, b). They cleaved at a phosphodiester immediately 3′ to a single ribonucleotide embedded within a DNA substrate (Fig. 1a, b) but were either unable to cleave when extended RNA of the same sequence replaced the original DNA substrate (Breaker and Joyce 1994, 1995) or did cleave but with significantly lowered rates (Roth and Breaker 1998).
Systematic evolution of ligands by exponential enrichment (SELEX) strategies to identify RNA-cleaving deoxyribozymes (red RNA, green potential deoxyribozyme). a General four-step selection strategy to enrich functional deoxyribozymes. b First approach used to identify the E2 deoxyribozyme. c Type of approach that Santoro and Joyce (1997) employed to isolate the 10–23 and 8–17 deoxyribozyme (modified after Silverman 2005)
Examples of various RNA-cleaving deoxyribozymes, which perform in trans (red RNA, green DNA, arrows cleavage sites). a, b Two of the first generation of Pb2+-dependent deoxyribozymes (Breaker and Joyce 1994, 1995). c, d The most frequently used deoxyribozymes 10–23 and 8–17 (Santoro and Joyce 1997). e, f Later developed deoxyribozymes, of which f does not require divalent cations (Feldman and Sen 2001; Roth and Breaker 1998). Note that the respective names and the relevant references are given below each deoxyribozyme (modified after Baum and Silverman 2008)
The next attempt was carried out by another group, Faulhammer and Famulok (1996), who were able to identify a Ca2+-dependent deoxyribozyme, Mg5, in spite of Ca2+ never being part of the selection process. However, this deoxyribozyme too was unable to cleave within an extended RNA sequence.
To tackle this problem, Santoro and Joyce (1997) generated a library of approximately 1014 single-stranded DNA molecules, in which each contained a 5′ biotin moiety followed (in a 5′→3′ direction) by a short oligodeoxynucleotide spacer, 12 target ribonucleotides (RNA sequence to be cleaved) and 50 random deoxynucleotides (potential DNA enzymes). The random region was surrounded by fixed-sequence deoxynucleotides as sites for primer hybridization (Fig. 1a, c). These molecules were applied to a streptavidin-coated solid support and eluted with a solution containing 10 mM MgCl2 at pH 7.5 and 37°C. Molecules that cleaved an RNA phosphoester within a small fraction of the bound molecule released the 3′ cleavage product into the eluate. The released material was recovered and amplified by the polymerase chain reaction (PCR). The PCR pool was then subject to generate a progeny population of DNA molecules, which were enriched for the desired population. Two sides in the target RNA sequence emerged to be hot-spots for cleavage, one during amplification round 6–8 and the other during round 9–10. After the eighth and tenth rounds, the enriched sequences were cloned and analyzed in detail. From a total of 62 clones, two motifs (8–17 and 10–23, Fig. 2c, d) showed extensive Watson-Crick pairing to the target RNA sequence located upstream and downstream of the cleavage site. The names of two deoxyribozymes were coined based on the 17th or 23rd clone of the 8th or 10th cycle of the in vitro selection process. The catalytic core of the 8–17 and 10–23 enzymes was located between the two substrate-binding arms that contained 13 and 15 deoxynucleotides, respectively (Santoro and Joyce 1997). Both deoxyribozymes required divalent metal ions such as Ca2+, Mg2+, Mn2+, Zn2+, or Cu2+ to cleave the target mRNA. The 8–17 DNA enzyme contained a short internal stem-loop (3 base pairs, at least two being G-C) followed by an unpaired region of 4–5 nucleotides. In contrast, the 10–23 DNA enzyme contained an unpaired loop structure where the eighth nucleotide position of the catalytic core was either a T, C, or A. The highest level of activity was provided with a T at this position. Apart from this, the entire loop sequence was intolerant to any changes (Sugimoto et al. 1999; Li et al. 2000).
Around the same time, Geyer and Sen (1997) identified an RNA-cleaving deoxyribozyme, Na8 that worked independently of divalent metal ions but it was able to increase RNA cleavage 108-fold. Monovalent ions such as Na+ were the effective cofactors. From the same laboratory, Feldman and Sen (2001) published a deoxyribozyme that preferred several unpaired ribonucleotides at the cleavage site. This newly formed bipartite I deoxyribozyme (12–17, Fig. 2e) had, however, an absolute requirement for Mg2+ or certain other divalent metal salts such as Mn2+, Zn2+ and Cu2+ for cleavage.
Roth and Breaker (1998) presented several histidine-dependent deoxyribozymes (HD2, Fig. 2f). To ensure the absence of divalent ions during the selection process, EDTA was added. HD2 required, in addition, K+ and the imidazole group from the histidine. This imidazole group most likely accelerated the cleavage reaction by facilitating deprotonation of the target site, the 2′ hydroxyl group.
The latest identification of a deoxyribozyme with broad pH tolerance by in vitro selection was performed by Xiao et al. (2010). The 9NL27 deoxyribozyme is one of several 10MD5 deoxyribozyme variants that enhance the hydrolysis of an uncatalyzed P-O bond at a rate of 1012. The deoxyribozymes 9NL27 and 10MD5 have comparable activities in the presence of a mixture of 1 mM Zn2+ and 20 mM Mn2+ at pH 7.5, whereas 10MD5 is essentially inactive when only 1 mM Zn2+ is used. In contrast, 9NL27 shows only a three-fold reduction under these conditions.
Digestion mechanism
One factor that influences the dissociation rate of deoxyribozymes to its substrate is the length of the binding arms, which form Watson-Crick base pairs with the target mRNA upstream and downstream of the cleavage site. Early studies by Sun and colleagues (1999) showed that a deoxyribozyme with a sequence length of 10 nucleotides of each arm (arm I, left site and arm II, right side of the deoxyribozyme, Fig. 3) performed best using a target sequence to human papilloma virus (HPV 16 E7), whereas deoxyribozymes with an arm I and arm II length combination of 8/10 or 6/10 performed better with a target sequence to c-myc. When the arm length of a deoxyribozyme to HPV 16 E7 was reduced to 6/10, a strong reduction was observed, whereas changes to 10/4 or 4/10 led either to an almost undetectable cleavage activity to HPV 16 E7 or strong reduction to c-myc (Cairns et al. 2000). Notably, when both binding arms are too long, dissociation of the target mRNA does not occur. Thus, only an antisense effect can be observed. When designing a deoxyribozyme, the length of the hybridization arms should be between 7 and 10 nucleotides on each side (Santoro and Joyce 1997) to secure cleavage and release as shown by Choi et al. (2010) for an arm I/armII length ratio of 10/10 and by Yang et al. (2009) for a ratio of 7/9.
Detailed labeling of the 10–23 deoxyribozyme after binding to a RNA substrate (red RNA, green DNA, arrow cleavage site). The 15-base sequence of the conserved catalytic core is numbered in the 5′→3′ orientation from 1 to 15. The binding domains on the 5′ and 3′ sides of the catalytic domain are designated arm I and arm II, respectively (modified after Cairns et al. 2000)
Digestion by the 10–23 deoxyribozyme requires a dinucleotide combination of G-C, G-U, A-C, or A-U in the target mRNA sequence, whereas for digestion by the 8–17 deoxyribozyme, solely A-G is sufficient (Fig. 2c, d). Hence, the 10–23 deoxyribozyme has a broader target range with a substrate requirement of an unpaired purine (A or G) and paired pyrimidine (C or U). This is ideal, because the more sites tested in long-folded mRNA, the greater the likelihood of finding highly accessible and cleavable sites (Cairns et al. 1999). The best efficiency of substrate cleavage was observed when the target site consisted in an A and U for the 10–23 deoxyribozyme (Santoro and Joyce 1997) and, in descending order, followed rAU=rGU≥rGrC>>rArC. To increase the activity, in particular, to the last two dinucleotide combinations (GC and AC), a substitution of dG with deoxyinosine (dI) is efficient. Thus, the three hydrogen bonds between rC-dG are converted to two hydrogen bonds between rC-dI, which improves the biological activity of the deoxyribozymes (Cairns et al. 2003).
Finally, cleavage occurs at the 3′ side of a single unpaired ribonucleotide, preferably the purine that is followed by the paired pyrimidine within an all-RNA substrate (Santoro and Joyce 1997). Hereby, the deoxyribozyme bends the substrate away from the cleavage point, exposing the reactive site and buckling its own catalytic core (Kenward and Dorfman 2009). Then, RNA cleavage is catalyzed through transesterification when the oxygen of a MO- (M = metal ion) or OH- group serves as a nucleophile to attack the phosphorus center of the adjacent phosphoester linkage in an SN2-like reaction. In other words, the divalent cations serve as a Lewis base or metal hydroxide, generating an end-product of 2′,3′-cyclic phosphate and 5′-hydroxyl RNA termini (Fig. 4). Afterwards, the catalytic core of the deoxyribozyme unwinds and the complex extends.
Representation of two hypothetical mechanisms for the catalysis of RNA cleavage by the 10–23 deoxyribozyme with metal cations (M+) such as Ca2+, Mg2+, Mn2+, Zn2+ and Co2+. a Mechanism involving a divalent metal hydroxide that functions as a general base (B). This base deprotonates the 2′-OH group. b Mechanism involving a divalent metal cation that functions as a Lewis acid. A Lewis acid is an electron-pair acceptor that polarizes the 2′-OH bond and accelerates deprotonation. In both cases, the RNA is cleaved to a 2′-3′ cyclic phosphate and 5′-hydroxyl termini with a metal cation and oxidized base or with a metal cation associated with H2O, respectively (modified after Santoro and Joyce 1998)
Modifications of deoxyribozymes
The origin of modifications for knockdown agents, which are prone to nucleolytic degradation in body fluids, started during antisense research. Thus, chemical modifications not only enhance bioactivity but also lower toxicity and increase target affinity (Kurreck 2003). To prevent deoxyribozyme digestion through serum nucleases and, at the same time, to increase the efficiency of the enzyme, the following modifications are the most frequently used: (1) phosphorothioate linkage, (2) 2′-O-methyl nucleotides, (3) 3′-terminal nucleotide inversions such as thymidine at the 3′ end and (4) locked nucleic acids (Fig. 5).
Modifications to increase stability and/or efficacy of deoxyribozymes (bold typeface denotes the modification). a Phosphorothioate oligonucleotides (PTO) replaces an oxygen atom with a sulfur atom. b A 2′-O-methyl group substitutes the hydrogen at the 2′-deoxyribose position. c Locked nucleic acids (LNA) are RNA-based nucleotides connecting the 2′-oxygen with the 4′-carbon. d The 3′-3′-inverted thymidine (T) increases the stability of the deoxyribozyme against nucleases. e N3′-P5′ phosphoramidate (NP) enhances the affinity to the single-stranded RNA substrate. f Ado-DNAzyme I contains a regulatory sequence that is able to bind adenosine (A). g Anthraquinone (t-Anth) is tethered between A15 and the binding arm II (summarized data from Takahashi et al. 2000; Wang et al. 2002; Schubert and Kurreck 2004, Asanuma et al. 2006)
Phosphorothioate oligonucleotides
Here, one of the non-bridging oxygen atoms in the phosphodiester bond is replaced by sulfur. Introduction of a small number of phosphorothioate oligonucleotides (PTOs), e.g., three at the 3′ end, rendered greater stability to the deoxyribozyme without influencing the efficacy (Sioud and Leirdal 2000). In a study by Sun et al. (1999), the introduction of phosphorothioate (PS) bonds into the hybridization arms and into the pyrimidine residues of the catalytic core selectively inhibited human glioma cell growth by up to 90%. However, Schubert et al. (2003) observed a deceleration of the cleavage rate to one-fifth when PS linkages were introduced throughout the entire binding arms; this might be attributable to a decrease in target affinity. In such cases, potential toxic side effects might also arise (for a review, see Levin 1999).
2′-O-methyl nucleotide
The introduction of methylphosphonates into deoxyribozyme backbones increases the relative hydrophobicity and eases diffusion across membranes (Shoji et al. 1991). After the introduction of two 2′-O-methyl at the 5′ and 3′ end or four 2′-O-methyl RNA monomers as end-blocks into a deoxyribozyme, a two-fold or six-fold increase, respectively, of the original cleavage rate was obtained (Warashina et al. 1999; Schubert et al. 2003).
Locked nucleic acids
These are ribonucleotides containing a methylene bridge that connects the 2′-oxygen of the ribose with the 4′-carbon (for a review, see Orum and Wengel 2001). Incorporation of locked nucleic acids (LNAs) into the binding arms improves the hybridization potential of several DNA enzymes, enhances the folding of the catalytic loop (Vester et al. 2006) and induces a conformational change toward the A-type helix (Bondensgaard et al. 2000).
Inverted nucleotide
Sioud and Leirdal 2000 introduced a 3′-3′-inverted thymidine into a deoxyribozyme and achieved a six-fold increase in stability with sustained cleavage activity, whereas others (Sun et al. 1999) have observed a ten-fold enhancement in serum stability. In combination with the arm length of the deoxyribozymes, Sun et al. (1999) have shown that an inverted thymidine influences the cleavage rate, ranging from a slight loss of efficiency to a four-fold increase of activity. In contrast, Schubert et al. (2003) has not been able to confirm this observation.
Further modifications of deoxyribozymes (Fig. 5), which increase the catalytic activity but are less common, are listed below:
-
N3′-P5′ phosphoramidate The N3′-P5′ phosphoramidate (NP) modification showed an increase of about two-fold in the relative efficiency of a deoxyribozyme to the translation initiation region of influenza A virus (Takahashi et al. 2000)
-
Adenosine binding To regulate the binding of a deoxyribozyme to its target mRNA, (1) a regulator oligonucleotide and adenosine (Ado-DNAzyme I) or (2) adenosine alone (Ado-DNAzyme II) is required (Fig. 5). By adding adenosine to Ado-DNAzyme I, the catalysis of the 10–23 and 8–17 deoxyribozyme can be increased by up to 35-fold (Wang et al. 2002)
-
Intercalator tethering When the intercalator, anthraquinone (t-anth), is tethered via D-threoninol directly through an amide bond between position A15 and the binding arm II, an eight-fold increase in activation is obtained (Asanuma et al. 2006).
Other modifications are focusing on the catalytic core of the 10–23 deoxyribozyme; the core is sensitive to any changes and should be preserved. In particular, positions G1, G2, T4, G6 and G14 (Fig. 3) are highly conserved and the substitution with any other base leads to the complete loss of catalytic activity. Known exceptions are: a nucleotide replacement at position 8, the least conserved base, from a T→N (N = C, G, A) and another at positions 7–12 by any naturally occurring nucleotides. Further substitutions are possible at position 14 from a G→A and at position 15 from an A→G, without severe effects (Joyce 2001; Zaborowska et al. 2002). From these results, one can conclude that nucleotides at positions 1–6 and 13–14 are directly involved in forming the catalytic center. Curiously, Schubert et al. (2003) have demonstrated that modifications with 2′-O-methyl groups at positions 2, 7, 8, 11, 14 and 15 do not interfere with the correct folding of the catalytic centre and even double the original value of its catalytic rate.
Finally, alteration of the deoxyribozyme size (17 to 33 nucleotides long) and chemistry do not affect the degree of uptake into cells in culture (Dass et al. 2002). For internalization, deoxyribozymes do not need support through electroporation, transfection, or infection.
Cellular uptake
In spite of the uptake of modified single-stranded DNA molecules having been widely investigated, some mechanistic aspects refuse to give up their secrets. DNA molecules that are associated with the cell membrane are internalized into the cytoplasm by cell surface receptors such as CD4 (Yakubov et al. 1993) and gp 120 (Stein et al. 1993) with the involvement of protein kinase C (PKC), which are unspecifically used as free-riders via receptor-mediated endocytosis. Alternatively, pinocytosis and absorptive endocytosis might also play an important role in oligonucleotide (ODN) uptake (Patil et al. 2004). Which of these mechanisms is used depends on factors such as ODN chemistry, length, conformation and concentration, cell type, stage of cell cycle, degree of cell differentiation and intracellular conditions or cell environment (e.g., pH and cation concentration; Akhtar et al. 2000). Hence, no universal mechanism that conclusively explains the internalization process has been identified. After uptake, when labeled, the DNA molecules appear in a characteristic punctated cytoplasmic distribution (Loke et al. 1989; Grimpe and Silver 2004). The fate of the DNA molecule depends on its localization into either endosomes or lysosomes. Lysosome membranes contain proton (hydrogen) pumps, which change the inside pH to 4.5-5. Such conditions not only promote acidic hydrolysis but also activate lysosomal enzymes that rapidly degrade the ODNs. In contrast, endosomes keep a neutral pH in their lumen so that no degradation of the ODNs occurs and hence, the ODNs are ready to work. The way in which the DNA molecule escapes the lysosomes or endosomes is, once again, unclear. A likely mechanism for penetrating these compartments is the conformational change of membrane proteins to destabilize the membrane, as occurs with bacterial toxins (Farahbahksch et al. 1987) and viruses (White et al. 1983). After being released into the cytoplasm, the DNA molecules are reported to become localized (1) predominantly in the cytoplasm or (2) within the nucleus (Zamecnik and Stephenson 1978; Cerruzi et al. 1990; Chin et al. 1990; Leonetti et al. 1991).
In conclusion, mention should be made of the special care that has to be taken when selecting cells for investigations. An often-observed problem is encountered on the utilization of cloned cell lines when using deoxribozymes or other knockdown agents: frequently, these cell lines show limited or no uptake of the agent, whereas primary cells show good to excellent internalization. Concerning the CNS, the author has successfully used hippocampal slice cultures (Grimpe and Silver 2004), primary astrocytes (Grimpe and Silver 2004) and primary retinal ganglion cells (Ries et al. 2007) with high efficiency of the internalization of deoxyribozymes. Schwann cells from the PNS also exhibit high efficiency (Grimpe et al. 2005), whereas dorsal root ganglion cells fail to internalize a deoxyribozyme (data not shown). For cloned cell lines, the aid of transfection reagents, electroporation, or infection is necessary for internalization, whereas primary cells do not need them. When such enhancing techniques are used on primary cells, the uptake of the ODNs, however, is increased. Laboratories that are newly interested in this research field will probably make the mistake of initially selecting the wrong cell type and will experience a lack of effect from their agents. In a further observation, the simple administration of chemically stabilized deoxyribozymes in a saline solution led to efficient uptake and biological effects in vivo. This finding points to unknown uptake mechanisms that occur inside organisms and that are not encountered in cell culture as yet (Schubert and Kurreck 2004). To circumvent these problems, Li et al. (2010) have used a single-stranded DNA expression vector that generates a 10–23 deoxyribozyme under the control of two separated promoters.
Therapeutic approaches
Nucleic-acid-based knockdown technologies such as deoxyribozymes, ribozymes, short interfering RNA (siRNA) and antisense ODNs depend on the accessibility of the target site. To identify suitable sites along the mRNA, theoretical approaches such as the MFOLD program (http://unafold.math.rpi.edu/) have been developed. Although the MFOLD program predicts RNA secondary structure based on free-energy calculations (Walter et al. 1994; Amarzguioui et al. 2000), interactions with proteins such as single-stranded proteins, to which every mRNA is attached, are excluded from analysis. Therefore, mRNA targeting is still a relatively random affair in which several agents are tested until an accessible site is identified.
The above-mentioned knockdown technologies have widespread applications in the fields of human immunodeficiency virus (HIV), cancer, influenza, hepatitis, asthma and spinal cord injury (SCI) research but the mechanism between these various technologies is quite different. Deoxyribozymes are non-naturally occurring, whereas ribozymes, in contrast, are single-stranded natural RNA catalysts. Both require mono- or divalent ions. siRNA molecules are small double-stranded RNA molecules with a 3′ overhang of one nucleotide on each side, whereas antisense ODNs are small single-stranded DNA molecules. The last two utilize the cellular machinery for the sequence-specific silencing process. siRNA molecules are incorporated into the RNA-induced silencing complex (RISC), which binds to the mRNA of interest and stimulates an mRNA degradation mechanism, such as nuclease activity, which leads to the silencing of the particular mRNA (Bertrand et al. 2002; McManus and Sharp 2002; Scherr et al. 2003; Kim and Rossi 2007). Antisense ODNs, however, bind stoichiometricly to the target mRNA and interfere with gene expression or other mRNA-dependent cellular mechanisms. In the presence of RNAse H, they induce an enzymatic degradation of the target mRNA (S.T. Crooke et al. 1995). The therapeutic applications of these four knockdown agents will be discussed below.
Deoxyribozymes
Based on its catalytic efficiency of 3.2×108M−1min−1, which is comparable to those of naturally occurring ribozymes, only the 10–23 deoxyribozyme has been applied in vivo to inactivate target mRNA molecules (Santoro and Joyce 1997). This efficiency is near-catalytic “perfection”, consistent with the Albery and Knowles definition, in which the reaction rate is limited only by the rate of diffusional encounter between the enzyme and substrate (Albery and Knowles 1976). Therapeutic use of DNA-based over RNA-based technologies is predicated on the absence of a 2′-OH group at each phosphodiester linkage, which makes DNA ∼100.000-fold more stable to hydrolysis than RNA under physiological conditions (Li and Breaker 1999). In other words, the half-life of an unmodified deoxyribozyme in human serum has been measured to be about 2 h compared with <1 min for hammerhead ribozymes (Schubert and Kurreck 2004). Further support for DNA-based technology comes from the finding that a DNA:RNA duplex tends to dissociate more easily than an RNA:RNA duplex. This means that mismatches in the hybridization arms, which might occur during binding to nontarget mRNAs, inhibit cleavage activity and increase dissociation from the target molecule (for a review, see Shaw and Arya 2008). Furthermore, the cleavage rates with long RNA molecules are several orders of magnitude lower than those with short substrate molecules (Thomson et al. 1996). Hence, when dealing with long-sequence targets, as likely in biological systems, a deoxyribozyme should be more active and potentially more selective than a ribozyme. As Table 1 shows, deoxyribozymes have been investigated with a variety of molecules. Their application to neurological disorders, however, is limited. The two companies that have been founded to date, namely the US-based CytoGenix (Tan et al. 2004) and the Germany-based Sterna Biologicals (Sel et al. 2008), test deoxyribozymes of the 10–23 type in preclinical trials of cancer, viral infections diseases such as influenza, small pox and HIV and asthma research. Whereas CytoGenix have generated a mammalian single-stranded DNA expression vector from which a 10–23 deoxyribozymes is expressed (called SynDNA), Sterna Biologicals administers intranasally a 10–23 deoxyriboyzme modified by an inverted 3′-3′-thymidine (Fig. 5d).
Ribozymes
The most frequently described and investigated ribozyme with therapeutic potential is the small hammerhead ribozyme (Birikh et al. 1997) followed by the hairpin ribozyme (Fig. 6). Further ribozymes or self-cleaving RNAs are the rRNA intervening sequence from Tetrahymena thermophila (Kruger et al. 1982), RNase P (Guerrier-Takada et al. 1983), hepatitis delta virus (Wu et al. 1989), transcript from a Neurospora crassa mitochondrial DNA plasmid (Saville and Collins 1990) and plant pathogenic RNAs (viroids, satellite RNAs, virusoids; Diener 1989). The hammerhead ribozyme requires a recognition sequence of NUH for cleavage of the target mRNA, where N is any nucleotide and H equals A, C, or U. Cleavage occurs between U and H (James and Turner 1997). In contrast, the hairpin ribozyme has a sequence requirement of BN/GUC, where “/” is the site of cleavage and B equals G, U, or C (Hampel 1998). For clinical use, efforts have been made to reduce the number of nucleotides in the binding arms and within the catalytic loop structure without compromising the cleavage ability or specificity of the hammerhead ribozyme. For most efficient cleavage, the 5′-arm requires 5–6 nucleotides, whereas the 3′-arm, which is the most crucial in this respect, has no limitation in the number of nucleotides, as long as it also contains a minimum of 5 or 6 bases. A reduction in the catalytic loop and the insertion of DNA, which leads to chimeric miniribozymes or minizymes (Fig. 6), results in faster cleavage of longer RNA substrates, at least in vitro. Here, the binding arms of miniribozymes or minizymes should rather have a length of around 10 nucleotides (Hendry et al. 1998). The actual cleavage reaction of ribozymes occurs on a susceptible 3′,5′-phosphodiester linkage leading to the formation of a 2′,3′-cyclic phosphodiester and 5′ hydroxyl group (Cech 1987), which is similar to the mechanism used by deoxyribozymes. During this process, the ribozyme requires the presence of Mg2+. Crystallization experiments have revealed that Mg2+ not only assists in RNA folding but also participates directly in the cleavage mechanism (Dahm and Uhlenbeck 1991; Pley et al. 1993).
siRNA
This knockdown technology has been the most frequently used during the last two decades, with high expectations for therapeutic application. A plethora of publications has focused on the mechanism of siRNA gene silencing, the delivery of the siRNA agent, its therapeutic utilization and any unwanted side-effects. New online tools provide effective design algorithms, which are based on a combination of mRNA target sequence, secondary structures and siRNA duplex end-stabilities and aim to minimize potential sequence-dependent off-target effects. Most of the proposed clinical applications of RNA interference (RNAi) incorporate chemically synthesized 21-nt siRNA duplexes that have two-nucletotide 3′ overhangs. When longer siRNAs (27 nucleotides) are used, a lower concentration is required for silencing the target. These agents then contain an asymmetrical 3′ overhang with two nucleotides and one blunt end. Here, the 3′overhang is recognized by Dicer for processing (Amarzguioui et al. 2005). Off-target effects seem to be avoidable when the complementary region of the siRNA is localized within the open reading frame evading the 3′ untranslated region (for a review, see Jackson and Linsley 2010). To elude a type I interferon response of double-stranded RNA agents, which are longer than 30 base pairs, or of double-stranded and single-stranded RNA agents via Toll-like receptors, the following immunostimulatory sequence motifs should be avoided: 5′-GUCCUUCAA-3′ and 5′-UGUGU-3′ (Hornung et al. 2005; Judge et al. 2005).
Various delivery strategies to guide the therapeutic siRNAs to their specific location have been utilized. For example, they have been coupled to antibody fragments (Song et al. 2005), aptamers (McNamara et al. 2006), or packaged into nanoparticles coated with receptor-targeting ligands (Hu-Lieskovan et al. 2005), whereby the siRNA is incorporated through endocytosis. Modifications with the 2′-O-methyl group not only avoid interferon induction (Morrissey et al. 2005) but also, when staggered between the two siRNA strands, seem to provide an optimal balance between retention of RNAi potency and protection against degradation (Czauderna et al. 2003). A summary on the therapeutic targets of siRNA agents is provided by Koutsillieri et al. (2007) and Lares et al. (2010).
In light of all the success achieved with siRNA and the investments made by big pharma, the latest decisions of the pharmaceutical industry are surprising. Roche closed its three R&D facilities working on siRNA in December 2010 (after a total of $500 million had been invested during the previous three years) and Pfizer and Abbott announced that it had no further interest in this technology. Novartis also showed reduced enthusiasm by ending a 5-year partnership with Alnylam at the end of 2010. However, Novartis will finish its program including 31 siRNAs, on which they are working internally (Pollak 2011; Schmidt 2011).
Antisense ODNs
The trailblazer of all knockdown agents is antisense technology. Our long-time experience with this technique provides the necessary foundation for the design of efficacious and specific ODNs. In spite of the size of the ODN being important and leaving little leeway, a hard-and-fast rule of a right-sized ODN does not exist; hence, it must be determined by empirical means. Some ODNs as short as 12 nucleotides have been found to provide a good discrimination of the target gene expression, whereas in other cases, a 16-base long ODN shows no selectivity. If an ODN is too short, it will not be selective at all but if it is too long, it will not be accessible to the tissue and will be difficult to work with. Control ODNs are essential for every experiment utilizing this technique. Hence, “mismatch ODNs”, which are scrambled antisense sequences, with the potential of keeping the same GC contents as their specifically working counterpart, are often used. Another control is the utilization of “mixed base ODNs”, which are sense sequences that contain 4 to 5 scrambled bases on their 3′ and 5′ ends. Pure sense controls are not to be recommended, because antisense mechanisms are involved in naturally occurring gene regulation such as for fibroblast growth factor-2 (FGF-2). Here, sense and antisense transcripts form double-stranded RNA duplexes in vivo. With the administration of a sense ODN to the antisense transcript, one generates a control that has biological activity and produces physiological effects that create confusing or even misleading results. Other common types of control involved “randomized” and “reverse ODNs”, in which the first is obtained by mixing up the bases of the antisense ODN and the latter by reversing the antisense sequence with respect to its 5′-3′ orientation. Moreover, the latter is unable to hybridize to the antisense mRNA target (Leslie et al. 1999). Needless to say, it is essential to analyze all antisense and control sequences for their specificity through a BLAST search.
For the clinical utilization of antisense ODNs, S.T. Crooke (1995), the founder, chairman and CEO of Isis Pharmaceuticals, the company that generated the first Food and Drink Administration (FDA)-approved an antisense drug in 1998, namely Vitravene, targeting cytomegalovirus retinitis in HIV-positive patients, eloquently summarized the pharmacokinetics of antisense PTOs. He states that antisense PTOs are readily absorbed from a variety of administration routes such as intramuscular, subcutaneous, intradermal, or intraperitoneal applications. These PTOs reach their maximum plasma concentration within 15–30 min. Distribution occurs rapidly and broadly to the peripheral tissue with accumulation of most of the drug in the liver and kidney without crossing the blood–brain barrier. Clearance of the antisense PTOs after being internalized into the cell takes place slowly (elimination half-life: 40–50 h) by slow metabolism (half-life: ∼24 h) in all tested organs. S.T. Crooke and colleagues (R.M. Crooke et al. 1995; Cossum et al. 1993, 1994; Sands et al. 1994; Agrawal et al. 1991) have observed minimal differences between species and between different sequences from the above-described statements. Thus, the in vivo delivery of antisense PTOs to peripheral tissues has proved to be relatively facile. In conclusion, Crook writes, “In animals and humans, perhaps to the surprise of some, antisense PTOs have displayed excellent parenteral pharmacokinetic properties concentrating in a number of peripheral tissues, including the liver, kidney, bone marrow, skeletal muscle and skin. Thus, specialized drug delivery systems have not been required to show potent in vivo activities.” Today, he adds, “Despite the early success, all the necessary elements were not in place at that time to make multiple products. Due to the worldwide shrinkage of the drug market in 2004, when big pharma experienced losses in the billions, Vitravene was discontinued because its patient group was too small.” Raymond Warrell, chairman and CEO of Genta adds, “If you’re going to have an effect with a single antisense agent, which attacks only a single gene product, you have to be sure that your target has an absolutely central role in the complex biology of the disease you’re trying to treat.” An example is the Isis anti-PKCα drug, which can be given at high doses as its side-effects are modest compared with those of other anticancer agents. “But it failed because PKCα was a bad choice of target”, says S.T. Crooke. “It’s just not very significant in the malignancies that we looked at” (Jones 2011). The revival of antisense drugs can be observed based on the number of licenses that the big pharmaceutical companies have distributed in the last two years (Pollak 2011). Not only target specificity but also safe treatment requires the investigation of the liver and kidney function through blood analyses, such as a complete blood count (CBC) and chemistry. Furthermore, the slow administration of antisense PTOs, in particular when using the systemic route, is essential in a safe treatment paradigm. In summary, Table 2 compares the advantages and disadvantages of the deoxyribozyme technologies with the other above-mentioned knockdown techniques.
Presently, 347 clinical studies exist for SCI and 348 for traumatic brain injury, whereas 15 recruitments to studies with siRNA (completed or active), two trials with ribozymes (completed or active) and 88 clinical trials (completed or active) based on antisense technology can be found on the FDA database (www.clinicaltrials.gov). The lack of deoxyribozymes trials demonstrates that this technology is still in its infancy. A combination of SCI or traumatic brain injury with one of these knockdown technologies has not been initiated.
Utilization of deoxyribozymes in axon regeneration
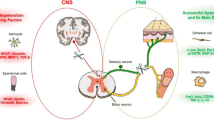
After trauma to the CNS, e.g., spinal cord injury, immediate early genes such as c-jun and also c-fos, nor-1 (Landry et al. 2006) and Zif268 (Densmore et al. 2010) are up- or down-regulated. If the blood-spinal cord-barrier opens after such an injury, a lesion scar forms, which, among other factors, prevents re-growth of the majority of severed axons. The scar consists in extracellular matrix molecules (ECMs), e.g., proteoglycans (PGs), laminins, fibronectin and collagen. Deoxyribozymes have been designed to some of these molecules and show a successful reduction of the specific mRNA in the CNS. These deoxyribozymes will now be discussed in more detail.
c-Jun
This basic region leucine-zipper transcription factor is activated through double phosphorylation by the c-Jun N-terminal kinase (JNK) on Ser-63 and Ser-73 within its transcriptional activation domain. JNK 3, one of three genes, is predominantly found in CNS neurons such as pyramidal neurons in the CA1, CA4 and the subiculum region of the hippocampus, whereas lower levels have been identified in the heart and testis. Maximum activation of JNK requires the phosphorylation of Thr and Tyr residues in the activation loop. If only the Thr residue via mitogen-activated protein kinase kinase 7 (MKK7) is phosphorylated, a significant increase of JNK3 can still be observed. In contrast, phosphorylation by MKK4 alone induces only low levels of JNK3. JNK activates, in addition to c-Jun, anti-activating transcription factor-2 (ATF-2), Jun B and Jun D (Ip and Davis 1998). Because JNK is involved in neuronal differentiation, apoptosis, neuronal diseases such as Alzheimer’s disease and Parkinson’s diseases and stroke, it is being pursued by several companies for CNS drug indications (Resnick and Fennell 2004).
The first study utilizing a deoxyribozyme to c-jun (Dz13) demonstrated that it blocked endothelial cell proliferation and migration. After injection of Dz13 into tumor cells, vascular endothelial growth factor (VEGF)-induced neovascularization in the rat cornea and B16 melanoma growth was inhibited (Zhang et al. 2004). The latest discoveries show that Dz13 is a potent inducer of caspase-2 (Dass et al. 2010a) and of the transcription factor, E2F1 (Dass et al. 2010b), based on off-target effects. Both molecules are involved in apoptotic processes, whereas the E2F class is additionally involved in mitosis, DNA replication, DNA damage checkpoints, DNA repair, development and differentiation. Further analysis has shown that ERK, Akt and p38 are also up-regulated after Dz13 administration (Dass et al. 2010b). The same group (Dass and Choong 2010) have found that systemic application of Dz13 represents no toxic effects for blood or solid tissues in adult or fetal mice, with only a slight hepatotoxicity being noticed by means of histology.
Laminin
Unlike most regions of the adult brain and spinal cord, the hippocampus possesses a remarkable capacity for continued axon growth and reformation of precise synaptic connections within the CNS. Indeed, new functional granule neurons with lengthy axons are generated in the hippocampus from a resident population of stem/progenitor cells throughout the adulthood of many species, including humans. Grimpe et al. 2002 describe a narrow sickle-shaped laminin γ1 chain pathway beginning in the hilus and extending along the CA3 pyramidal cell body layer (stratum pyramidal). Staining is also present in and around the portion of the apical dendrites closest to the cell body (stratum lucidum) but is lacking entirely in the basal and distal-most portions of the apical dendrites of the young CA3 pyramidal neurons. Thus, laminin staining is confined strictly within the stratum pyramidal and stratum lucidum, providing the newly generated granule neurons with a growth-promoting guidance highway for their mossy axons. O’Keefe and Nadel 1978 and Amaral and Dent 1981 have identified the identical terrain as a mossy fiber pathway.
In hippocampal slice cultures, the mossy fibers show the ability to regenerate after being lesioned at the hilus/CA3 region. However, when a deoxyribozyme to the laminin γ1 chain is administered, the laminin in this pathway is reduced and the mossy fibers lose their ability to regenerate. Instead, they are much shorter and take a meandering course. When the deoxyribozyme is washed out, laminin immunoreactivity returns and mossy fiber regeneration resumes (Grimpe et al. 2002).
Xylosyltransferase-1
In the last decade, PGs have been identified as ECM molecules that participate in the growth inhibitory environment that is formed after an injury in the spinal cord. Whether their glycosaminoglycan (GAG)-chains or their protein cores prevent the regeneration of severed axons is still controversial. However, prevention of the initiation of these GAG-chains, which begin primarily in the Golgi apparatus (Hoffmann et al. 1984), increases axon growth as demonstrated by Moon et al. (2001) and Bradbury et al. (2002). In the Golgi apparatus, the core proteins of PGs receive the long unbranched GAG-side chains. Chondroitin sulfate (CS)-, dermatan sulfate (DS)- and heparan sulfate (HS)-PGs all have a special link tetrasaccharide, composed of xylose, galactose, galactose and glucuronic acid. This link-molecule is attached to a serine hydroxyl side chain, embedded in a specific consensus peptide sequence at the core protein, to serve as a primer for polysaccharide growth. The first carbohydrate of the GAG-chains, namely xylose from UDP-xylose, is catalyzed by the chain-initiation enzyme UDP-D-xylosyltransfease (XT-1; Götting et al. 2000). Furthermore, sugars such as D-glucuronic acid (GlcA) and N-acetyl-D-galactosamine (GalNAc) are added by specific glycosyl transferases as disaccharide units individually to the link tetrasaccharide. While still in the Golgi compartment, many of the polymerized sugars are covalently modified by a sequential and coordinated series of reactions, such as sulfatation at the C2 position of GlcA and/or the C4 or C6 position of GalNAc residues. In principle, PGs have the potential for almost limitless heterogeneity regarding the number and types of GAG chains attached to even one single type of core protein.
In Grimpe and Silver (2004), the local administration of a deoxyribozyme to XT-1 reduced the presence of GAG chains, which normally create an inhibitory penumbra around the epicenter of spinal cord lesions. The reduction of fully glycosylated PGs allowed for the regeneration of microtransplanted adult sensory axons around and beyond the central core of the lesion. This result was confirmed in Hurtado et al. (2008), in which endogenous severed sensory axons were able to enter and grow into spinal cord tissue rostral of a peripheral nerve graft after deoxyribozyme to XT-1 treatment. In an untreated spinal cord, this entry and growth was stalled based on enhanced GAG-chain synthesis.
These examples demonstrate that deoxyribozymes are successful in reducing the mRNA of proteins. In addition, the number of deoxyribozymes provided in Table 1 demonstrates that their application is flourishing. Hopefully, more neuroscientists will recognize this technology as a fruitful and rewarding alternative compared with established techniques.
Bioinformatics
At the beginning of the 21st century, microarray experiments were introduced to the neurobiology field, in particular for the study of the pathophysiology of the injured spinal cord. With this approach, bioinformatics and statistical software have to a large extent found their way into the SCI community.
DNA-microarrays
Three phases after SCI are typically recognized: (1) the acute phase, (2) secondary tissue loss and (3) the chronic phase (Tator 1996, 1998). After immediate mechanical damage to the spinal nervous tissue in the acute phase (minutes to days), cell death (necrosis), a barrage of action potentials (spinal shock), hemorrhages and swelling occurs, leading to motor functional restrictions or loss. During the secondary phase (minutes to weeks), ischemic cell death, electrolytic shifts and edema continue and, in addition, lipid peroxidation, free-radical production, apoptosis and reactive gliosis occur. Within 24 h, neutrophils invade the spinal parenchyma, followed by lymphocytes, resulting in an increase in cytokines and chemokines, which contribute to the loss of tissue. At this time, inhibitory factors such as PGs are expressed and the scar gradually matures at the peri-lesion site. In the chronic phase (days to years), apoptosis continues, additional demyelination causes more conduction deficits and, in about 25% of all patients, tethering of the spinal cord occurs. In approximately 20% of all SCI patients, a cyst forms at the injury site, which usually continues to enlarge (syringomyelia; Hulsebosch 2002).
Starting with Farlow et al. (2000), several scientific groups began to analyze the changes in gene expression throughout these three phases in various PNS- and CNS-trauma and regeneration models. Up to the present day, a wide range of different approaches have been performed, some with rather specialized questions, such as those investigating two temporally distinct microglia-related gene clusters (Byrnes et al. 2006) or those analyzing the effect of N-methyl-D-aspartate receptor inhibition (Nesic et al. 2002) and others with broader questions as summarized in Bareyre and Schwab 2003. In spite of providing important new insights into the pathophysiology of the injured spinal cord, all these approaches have in common that only a relative small number of genes have been selected for further investigations. The sheer volume of data creates additional challenges. Dedicated software and database systems to store and organize the microarray data and images are required. A description of the experimental parameters (annotation) via the MIAME standard, normalization of the data and clustering techniques have proven valuable but require a new standard in statistical analysis. For the first time, data and text-mining methods, k-nearest neighbor classifications, support vector machines, machine learning algorithms and neural nets have found applications in the field. Kaiser and Nisenbaum (2003) have combined published data sets from five different microarray studies to examine global changes in nerve tissue gene expression. They have been able to extend the biological conclusions derived from each study.
Novel approach
The next logical step is to build upon all existing data to comprehend the complex process of events and, at the same time, to identify suitable target molecules for the design of new deoxyribozymes to prevent regeneration failure. Therefore, it will be essential to collect all existing information on genes and proteins found in publications and databases, to connect these data utilizing bioinformatics and to validate the computer-based results through hypothesis-driven experiments (Fig. 7). To provide such an overall picture of SCI, its underlying biochemical pathways and potential protein interactions and to lay hands on the sheer number of applied substances, Ries et al. (2007) have written a preliminary computer program suite, called the regeneration data mining project (RDMP), to build an in-silico protein interaction model, drawing both from natural language sources and from various databases available on-line. A large MySQL database provides the sequence, interaction, literature and other data records plus query capability. RDMP is able to collect, identify and generate a list of proteins involved in processes of axonal growth from abstracts that have been collected in PubMed and classified with the medical subject heading (MeSH) term “nerve regeneration” (Ries et al. 2007). MeSH is a controlled vocabulary maintained by the National Medical Library, which covers subjects in the medical domain. The RDMP recognizes protein names by a dictionary/rule-based method similar to that of Egorov et al. (2004). The dictionary has been extracted from the DEF field of the universal protein resource UniProt database (Emmert et al. 1994). A list of English stop words and the most frequent words used in natural language texts were obtained from MySQL v4.1, to which the letters from the alphabet were added. An example in Table 3 illustrates the method’s main steps. In this approach, publications build the foundation of the analysis, instead of microarray databases. The latter contain a high number of false-positive and false-negative results, which have to be identified and filtered out, whereas the review process by journals for manuscripts should assure the quality of the data.
With the prototypic program suite, Grimpe and colleagues were able to identify 942 proteins, which were being evaluated in the area of nerve regeneration at that time. When classified by using gene ontology terms, proteins from every field of biochemistry were found in our list (Ries et al. 2007). This confirmed the original hypotheses that SCI is based on complex events. It further demonstrates the challenges that one is facing in modern science. Within the 942 proteins, we indentified osteopontin. Further analysis determined a novel function for osteopontin and its receptor CD44 and for the laminin-CD44 interaction in neurite outgrowth of retinal ganglion cells, an event that remained elusive at that time (Ries et al. 2007). When continuing to use the 942 proteins as a seed to search the Biomolecular Interaction Network Database (BIND), the authors were able to generate a protein-protein (P-P) interactions map. By analyzing these P-P interactions, which were visualized by Cytoscape (Fig. 8), three molecules new to and hence uninvestigated in the field of SCI were identified. These proteins were:
-
(1)
Protein inhibitor of activated STAT (PIASγ): Function: Specific inhibitor of transcription factors. Interactions: with nuclear hormone receptors as a co-regulator (Moilanen et al. 1999; Kotaja et al. 2000), with a specific SUMO ligase to p53, c-Jun (Schmidt and Muller 2002; for a review, see Shuai and Liu 2005) and Smad7 (Imoto et al. 2003) and as a suppressor to regulate transforming growth factor (TGF)-β-mediated Smad3 activation (Imoto et al. 2003).
-
(2)
Son of sevenless (Sos): Interacts directly with mitogen-activated protein kinase, a protein from our seed list. This is a guanine nucleotide exchange factor (Boguski and McCormick 1993; Simon et al. 1991). Function: Regulates Ras activity (Downward 1992); Sos1 knockout results in embryonic lethality in mice, indicating its essential function for mammalian development (Wang et al. 1997). Localization: Human cells contain two SOS (hSos) genes that encode closely related ubiquitously expressed proteins (Chardin and Mattei 1994); they are normally localized in the cell cytosol but, in response to growth factor stimulation, they are recruited to the plasma membrane (Bar-Sagi 1994). Only one publication (Marciano et al. 2004), which describes the investigation of, among others, SOS during hippocampal apoptosis after traumatic brain injury, has surfaced so far.
-
(3)
SH3-GRB2: Src-homology-3 (SH3) domain, folding of SH3-domain-carrying proteins into a conserved secondary structure appears critical for binding to its ligand rather than for its primary structure (Feng et al. 1994; Yu et al. 1994). Functions: Signal transduction (for a review, see Pawson 1995), cell polarization (Chenevert et al. 1992), enzymatic activation (Leto et al. 1990; Volpp et al. 1989), transcriptional regulation (Bustelo et al. 1992; Giachino et al. 1997) and limb morphogenesis (Chan et al. 1996).
Small part of the protein interaction network generated by the regeneration data mining project and rendered by Cytoscape v2.5.0 (pink dots proteins, blue lines protein interaction). In the circle at the bottom of the image and magnified in the insert, several proteins are presented that have so far not been investigated after SCI, such as PIASγ (A) and SH3-domain-GRB-2-like 3 (B), whereas glial fibrillary acidic protein (C) is one of our 964 seed list proteins (created by B. Grimpe)
Real time quantitative PCR of injured spinal cords (n=3) normalized to β-actin revealed that PIASγ and SOS have their expression peak at 3 h after contusion, whereas the SH3-GRB2 peak occurs after 6 h (Fig. 9). None of these proteins have been investigated regarding their potential function after SCI, in spite of their importance being demonstrated during, for example, neuronal development.
Real time PCR was performed on contused spinal cords (n=3) and sham (n=2) operated animals in triplicate. RNA was isolated 1, 3, 6, 24 and 48 h after lesion. The normalized expression values of the genes (PIAS protein inhibitor of activated STAT, SOS son of sevenless, SH3 Src-homology-3) are presented on a logarithmic scale (created by B. Grimpe)
Furthermore, the prototypic program suite has enabled the authors to rank the proteins based on their number of publications (Table 4). The generated list projects the political importance of the respective proteins and the direction that is favored by the regeneration community in order for it to comprehend the processes after trauma rather than the scientific importance. Nevertheless, the scientific information received about these selected and extensively studied proteins will still be important for fitting together the pieces of the puzzle of regeneration failure.
Concluding remarks
Deoxyribozymes have great potential to serve as knockdown agents for a variety of neurobiological problems and for therapeutic purposes because of their easy administration, reliability, specificity and low production costs. When used thoughtfully and within its limits, this technology is especially suitable for in vivo approaches to manipulate the environment of the injured spinal cord or brain tissue. In combination with a bioinformatics approach to indentify suitable but less-well-studied target molecules, the way is paved for a better comprehension of the mechanisms that lead to regeneration failure of axons after CNS trauma and, ultimately, to finding a treatment for paralysis.
References
Ackermann JM, Kanugula S, Pegg AE (2005) DNAzyme-mediated silencing of ornithine decarboxylase. Biochemistry 44:2143–2152
Agrawal S, Temsamani J, Tang JY (1991) Pharmacokinetics, biodistribution and stability of oligodeoxynucleotide phosphorothioates in mice. Proc Natl Acad Sci USA 88:7595–7599
Akhtar S, Hughes MD, Khan A, Bibby M, Hussain M, Nawaz Q, Double J, Sayyed P (2000) The delivery of antisense therapeutics. Adv Drug Deliv Rev 44:3–21
Albery WJ, Knowles JR (1976) Evolution of enzyme function and the development of catalytic efficiency. Biochemistry 15:5631–5640
Amaral DG, Dent JA (1981) Development of the mossy fibers of the dentate gyrus. I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol 195:51–86
Amarzguioui M, Brede G, Babaie E, Grotli M, Sproat B, Prydz H (2000) Secondary structure prediction and in vitro accessibility of mRNA as tools in the selection of target sites for ribozymes. Nucleic Aids Res 28:4113–4124
Amarzguioui M, Rossi JJ, Kim D (2005) Approaches for chemically synthesized siRNA and vector-mediated RNAi. FEBS Lett 579:5974–5981
Asanuma H, Hayashi H, Zhao J, Liang X, Yamazawa A, Kuramochi T, Matsunaga D, Aiba Y, Kashida H, Komiyama M (2006) Enhancement of RNA cleavage activity of 10–23 DNAzyme by covalently introduced intercalator. Chem Commun (Camb) 2006:5062–5064
Bareyre FM, Schwab ME (2003) Inflammation, degradation and regeneration in the injured spinal cord: insights from DNA microarrays. Trends Neurosci 26:555–563
Bar-Sagi D (1994) The Sos (Son of sevenless) proteins. Trends Endocrinol Metab 5:165–169
Baum DA, Silverman SK (2008) Deoxyribozymes: useful DNA catalysts in vitro and in vivo. Cell Mol Life Sci 65:2156–2174
Bertrand JP, Pottier M, Vekris A, Opolon P, Maksimenko A, Malvy C (2002) Comparison of antisense oligonucleotides and siRNA in cell culture and in vivo. Biochem Biophys Res Commun 296:1000–1004
Berzal-Herranz A, Burke JM (1997) Ligation of RNA molecules by the hairpin ribozyme. Methods Mol Biol 74:349–355
Bhindi R, Khachigian LM, Lowe HC (2006) DNAzymes targeting the transcription factor Egr-1 reduce myocardial infarct size following ischemia-reperfusion in rats. J Thromb Haemost 4:1479–1483
Birikh KR, Heaton PA, Eckstein F (1997) The structure, function and application of the hammerhead ribozyme. Eur J Biochem 245:1–16
Boguski MS, McCormick F (1993) Proteins regulating Ras and its relatives. Nature 366:643–654
Bondensgaard K, Petersen M, Singh SK, Rajwanshi VK, Kumar R, Wengel J, Jacobsen JP (2000) Structural studies of LNA:RNA duplexes by NMR: conformations and implications for RNase H activity. Chem Eur J 6:2687–2695
Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB (2002) Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416:636–640
Breaker RR, Joyce GF (1994) A DNA enzyme that cleaves RNA. Chem Biol 1:233–229
Breaker RR, Joyce GF (1995) A DNA enzyme with Mg2+-dependent RNA phosphoesterase activity. Chem Biol 2:655–660
Bustelo XR, Ledbetter JA, Barbacid M (1992) Product of vav proto-oncogene defines a new class of tyrosine protein kinases substrates. Nature 356:68–71
Byrnes KR, Garay J, Di Giovanni S, De Biase A, Knoblach SM, Hoffman EP, Movsesyan V, Al F (2006) Expression of two temporally distinct microglia-related gene clusters after spinal cord injury. Glia 53:420–433
Cairns MJ, Hopkins TM, Witherington C, Wang L, Sun LQ (1999) Target site selection for an RNA-cleaving catalytic DNA. Nat Biotechnol 17:480–486
Cairns MJ, Hopkins TM, Witherington C, Sun LQ (2000) The influence of arm length asymmetry and base substitution on the activity of the 10–23 DNA enzyme. Antisense Nucleic Acid Drug Dev 10:323–332
Cairns MJ, King A, Sun LQ (2003) Optimization of the 10–23 DNAzyme-substrate pairing interactions enhanced RNA cleavage activity at purine-cytosine target sites. Nucleic Acids Res 31:2883–2889
Cech TR (1987) The chemistry of self-splicing RNA and RNA enzymes. Science 236:1532–1539
Cerruzi M, Draper K, Schwartz J (1990) Natural and phosphorothioated modified oligonucleotides exhibits a non-random cellular distribution. Nucleosides Nucleotides 9:679–695
Chan DC, Bedford MT, Leder P (1996) Formin binding proteins bear WWP/WW domains that bind praline rich peptides and functionally resemble SH3 domains. EMBO J 15:1045–1054
Chardin P, Mattei MG (1994) Chromosomal localization of two genes encoding human ras exchange factors: SOS1 maps to the 2p22→p16 regions and SOS2 to the 14p21→q21 region of the human genome. Cytogenet Cell Genet 66:68–69
Chen F, Li Z, Wang R, Liu B, Zeng Z, Zhang H, Zhang J (2004) Inhibition of ampicillin-resistant bacteria by novel mono-DNAzymes and di-DNAzyme targeted to β-lactamase mRNA. Oligonucleotides 14:80–89
Chenevert J, Corrado K, Bender A, Pringle J, Herskowitz I (1992) A yesst gene (BEM1) necessary for cell proliferation whose product contains two SH3 domains. Nature 356:77–79
Chin DJ, Green GA, Zon G, Szoka FC Jr, Straubinger RM (1990) Rapid nuclear accumulation of injected oligodeoxyribonucleotides. New Biol 2:1091–1100
Choi BR, Gwak J, Kwon HM, Oh S, Kim KP, Choi WH, Cho YH, Kim DE (2010) Oligodeoxyribozymes that cleave beta-catenin messenger RNA inhibit growth of colon cancer cells via reduction of beta-catenin response transcription. Mol Cancer Ther 9:1894–1902
Cossum PA, Sasmor H, Dellinger D, Truong L, Cummins L, Owens SR, Markham PM, Shea JP, Crooke ST (1993) Disposition of the 14 C-labeled phosphorothioate oligonucleotide, ISIS 2105, after intravenous administration to rats. J Pharmacol Exp Ther 267:1181–1190
Cossum PA, Truong L, Owens SR, Markham PM, Shea JP, Crooke ST (1994) Pharmacokinetics of a 14 C-labeled phosphorothioate oligonucleotide, ISIS 2105, after intradermal administration to rats. J Pharmacol Exp Ther 269:89–94
Crooke ST (1995) Delivery of oligonucleotides and polynucleotides. J Drug Targ 3:185–190
Crooke ST, Lemonidis KM, Neilson L, Griffey R, Lesnik EA, Monia BP (1995) Kinetic characterization of Escherichia coli RNase H1: cleavage of various antisense oligonucleotide-RNA duplexes. Biochem J 312:599–608
Crooke RM, Graham MJ, Cooke ME, Crooke ST (1995) In vitro pharmacokinetics of the phosphorothioate antisense oligonucleotide. J Pharmacol Exp Ther 275:462–473
Czauderna F, Fechtner M, Darnes S, Aygün H, Klippel A, Pronk GJ, Fiese K, Kaufmann J (2003) Structural variations and stabilising modifications of synthetic siRNAs in mammalian cells. Nucleic Acids Res 31:2705–2716
Dahm SC, Uhlenbeck OC (1991) Role of divalent metal ions in the hammerhead RNA cleavage reaction. Biochemistry 30:9464–9469
Dash BC, Banerjea AC (2004) Sequence-specific cleavage activities of DNA enzymes targeted against HIV-1 Gag and Nef regions. Oligonucleotides 14:41–47
Dass CR, Choong PF (2010) Sequence-related off-target effect of Dz13 against human tumor cells and safety in adult and fetal mice following systemic administration. Oligonucleotides 20:51–60
Dass CR, Saravolac EG, Li Y, Sun LQ (2002) Cellular uptake, distribution, and stability of 10–23 deoxyribozyme. Antisense Nucleic Acid Drug Dev 12:289–299
Dass CR, Galloway SJ, Choong PF (2010a) Dz13, a c-jun DNAzyme, is a potent inducer of caspase-2 activation. Oligonucleotides 20:137–146
Dass CR, Tan ML, Galloway SJ, Choong PF (2010b) Dz13 induces a cytotoxic stress response with upregulation of E2F1 in tumour cells metastasising to or from bone. Oligonucleotides 20:79–91
Densmore VS, Kalous A, Keast JR, Osborne PB (2010) Above-level mechanical hyperalgesia in rats develops after incomplete spinal cord injury but not after cord transection, and is reversed by amitriptyline, morphine and gabapentin. Pain 151:184–193
Diener TO (1989) Circular RNAs: relics of precellular evolution? Proc Natl Acad Scie USA 86:9370–9374
Downward J (1992) Regulatory mechanisms for ras proteins. Bioessays 14:177–184
Egorov S, Yuryey A, Daraselia N (2004) A simple and practical dictionary-based approach for identification of proteins in Medline abstracts. J Med Inform Assoc 11:174–178
Emmert DB, Stoehr PJ, Stoesser G, Cameron GN (1994) The European Bioinformatics Institute (EBI) databases. Nucleic Acids Res 26:3445–3449
Fahmy RG, Khachigian LM (2004) Locked nucleic acid modified DNA enzymes targeting early growth response-1 inhibit human vascular smooth muscle cell growth. Nucleic Acids Res 32:2281–2285
Fahmy RG, Waldman A, Zhang G, Mitchell A, Tedla N, Cai H, Geczy CR, Chesterman CN, Perry M, Khachigian LM (2006) Suppression of vascular permeability and inflammation by targeting of the transcription factor c-Jun. Nat Biotechnol 24:856–863
Farahbahksch ZT, Baldwin RL, Wisnieski BJ (1987) Effects of low pH on the conformation of Pseudomonas exotoxin A. J Biol Chem 262:2256–2261
Farlow DN, Vansant G, Cameron AA, Chang J, Khoh-Reiter S, Pham NL, Wu W, Sagara Y, Nicholls JG, Carlo DJ, Ill CR (2000) Gene expression monitoring for gene discovery in models of peripheral and central nervous system differentiation, regeneration, and trauma. J Cell Biochem 80:171–180
Faulhammer D, Famulok M (1996) The Ca2+ ion as a cofactor for a novel RNA-cleaving deoxyribozyme. Angew Chem Int Ed Engl 35:2837–2841
Feldman AR, Sen D (2001) A new and efficient DNA enzyme for the sequence-specific cleavage of RNA. J Mol Biol 313:283–294
Feng S, Chen JK, Yu H, Simon JA, Schreiber SL (1994) Two binding orientations for peptides to the Src SH3 domain: development of a general model for SH3-ligand interactions. Science 266:1241–1247
Geyer CR, Sen D (1997) Evidence for the metalcofactor independence of an RNA phophodiester-cleaving DNA enzyme. Chem Biol 4:579–593
Giachino C, Lantelme E, Lanzetti L, Saccone S, Valle G della, Migone N (1997) A novel SH3-containing human gene family preferentially expressed in the central nervous system. Genomics 41:427–434
Götting C, Kuhn J, Zahn R, Brinkmann T, Kleesiek K (2000) Molecular cloning and expression of human UDP-d-xylose:proteoglycan core proten beta-d-xylosyltransferase and its first isoform XT-II. J Mol Biol 304:517–528
Grimpe B, Silver J (2004) A novel DNA enzyme reduces glycosaminoglycan chains in the glial scar and allows microtransplanted dorsal root ganglia axons to regenerate beyond the lesions in the spinal cord. J Neurosci 24:1393–1397
Grimpe B, Dong S, Doller C, Temple K, Malouf AT, Silver J (2002) The critical role of basement membrane-independent laminin γ1 chain during axon regeneration in the CNS. J Neurosi 22:3144–3160
Grimpe B, Pressman Y, Lupa MD, Horn KP, Bunge MB, Silver J (2005) The role of proteoglycans in Schwann cell/astrocyte interactions and in regeneration failure at PNS/CNS interfaces. Mol Cell Neurosci 28:18–29
Guerrier-Takada C, Gardiner K, Marsh T, Pace N, Altman S (1983) The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell 35:847–857
Hampel A (1998) The hairpin ribozyme: discovery and development for gene therapy. In: Scanlon KJ (ed) Therapeutic application of ribozymes. Methods in molecular medicine, vol 11. Humana, Totowa, pp 17–28
Hendry P, Lockett TJ, McCall MJ (1998) Small efficient hammerhead ribozymes. In: Scanlon KJ (ed) Therapeutic application of ribozymes. Methods in molecular medicine, vol 11. Humana, Totowa, pp 1–15
Hjiantoniou E, Iseki S, Uney JB, Phylactou LA (2003) DNazyme-mediated cleavage of Twist transcripts and increase in cellular apoptosis. Biochem Biophys Res Commun 300:178–181
Hoffmann HP, Schwartz NB, Roden L, Prockop DJ (1984) Location of xylosyltransferase in the cisternae of the rough endoplasmic reticulum of embyonic cartilage cells. Connect Tissue Res 12:151–163
Hornung V, Guenthner-Biller M, Bourquin C, Ablasser A, Schlee M, Uematsu S, Noronha A, Manoharan M, Akira S, Fougerolles A de, Endres S, Hartmann G (2005) Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR 7. Nature Med 11:263–270
Hou W, Ni Q, Wo J, Li M, Liu K, Chen L, Hu Z, Liu R, Hu M (2006) Inhibition of hepatitis B virus X gene expression by 10–23 DNAzymes. Antiviral Res 72:190–196
Hou Z, Meng JR, Zhao JR, Hu BQ, Liu J, Yan XJ, Jia M, Luo XX (2007a) Inhibition of β-lactamase-mediated oxacillin resistance in Staphylococcus aureus by a deoxyribozyme. Acta Pharmacol Sin 28:1775–1782
Hou Z, Meng JR, Niu C, Wang HF, Liu J, Hu BQ, Jia M, Luo XX (2007b) Restoration of antibiotic susceptibility in methicillin-resistant Staphylococcus aureus by targeting mecR1 with a phosphorothioate deoxyribozyme. Clin Exp Pharmacol Physiol 34:1160–1164
Hu-Lieskovan S, Heidel JD, Bartlett DW, Davis ME, Triche TJ (2005) Sequence-specific knockdown of EWS-FLI1 by targeted, nonviral delivery of small interfering RNA inhibits tumor growth in a murine model of metastatic Ewing’s sarcoma. Cancer Res 65:8984–8992
Hulsebosch CE (2002) Recent advances in pathophysiology and treatment of spinal cord injury. Adv Physiol Educ 26:238–255
Hurtado A, Podini H, Oudega M, Grimpe B (2008) Deoxyribozyme-mediated knock down of xylosyltransferase-1 mRNA promotes sensory axon regeneration in the adult rat spinal cord. Brain 131:2596–2605
Imoto S, Sugiyama K, Muromoto R, Sato N, Yamamoto T, Matsuda T (2003) Regulation of transforming growth factor–β signalling by protein inhibitor of activated STAT, PIASγ through Smad3. J Biol Chem 278:34253–34258
Ip YT, Davis RJ (1998) Signal transduction by the c-Jun N-terminal kinase (JNK)—from inflammation to development. Curr Opin Cell Biol 10:205–219
Isaka Y, Nakamura H, Mizui M, Takabatake Y, Horio M, Kawachi H, Shimizu F, Imai E, Hori M (2004) DNAzyme for TGF-beta suppressed extracellular matrix accumulation in experimental glomerulonephritis. Kidney Int 66:586–590
Jackson AL, Linsley PS (2010) Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat Rev Drug Discov 9:57–67
Jakobsen MR, Haasnoot J, Wengel J, Berkhout B, Kjems J (2007) Efficient inhibition of HIV-1 expression by LNA modified antisense oligonucleotides and DNAzymes targeted to functionally selected binding sites. Retrovirology 4:29
James HA, Turner PC (1997) Ribozymes. Methods Mol Biol 74:1–9
Jones D (2011) The long march of antisense. Nat Rev Drug Discov 10:401–402
Joyce GF (2001) RNA cleavage by 10–23 DNA enzyme. Methods Enzymol 341:503–517
Judge AD, Sood V, Shaw JR, Fang D, McClintock K, MacLachlan I (2005) Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotechnol 23:457–462
Kabuli M, Yin JA, Tobal K (2004) Targeting PML/RARα transcript with DNAzymes results in reduction of proliferation and induction of apoptosis in APL cells. Hematol J 5:426–433
Kaiser S, Nisenbaum LK (2003) Evaluation of common gene expression patterns in the rat nervous system. Physiol Genomics 16:1–7
Kenward M, Dorfman KD (2009) Coarse-grained Brownian dynamics simulations of the 10–23 DNAzyme. Biophysical J 97:2785–2793
Kim DH, Rossi JJ (2007) Strategies to silence human diseases using RNA interference. Nat Rev Genet 8:173–184
Kotaja N, Aittomaki S, Silvednnoinen O, Palvimo JJ, Janne OA (2000) ARIP3 (androgen receptor-interacting protein3) and other PIAS (protein inhibitor of activated STAT) proteins differ in their ability to modulate steroid receptor-dependent transcriptional activation. Mol Endocrinol 14:1986–2000
Koutsillieri E, Rethwilm A, Scheller C (2007) The therapeutic potential of siRNA in gene therapy of neurodegenerative disorders. J Neural Transm Suppl 72:43–49
Kretschmer T, Nguyen DH, Beuerman RW, Happel LT, England JD, Tiel RL, Kline DG (2002) Painful neuromas: a potential role for a structural transmembrane protein, ankyrin G. J Neurosurg 97:1424–1431
Kruger K, Grabowski PJ, Zaug AJ, Sands J, Gottschling DE, Cech TR (1982) Self-splicing RNA: autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena. Cell 31:147–157
Kurreck J (2003) Antisense technologies: improvement through novel chemical modifications. Eur J Biochem 270:1628–1644
Landry ES, Rouillard C, Levesque D, Guertin PA (2006) Profile of immediate early gene expression in the lumbar spinal cord of low-thoracic paraplegic mice. Behav Neurosci 120:1384–1388
Lares MR, Rossi JJ, Ouellet DL (2010) RNAi and small interfering RNAs in human disease therapeutic applications. Trends Biotechnol 28:570–579
Leonetti JP, Mechti N, Degols G, Gagnor C, Lebleu B (1991) Intracellular distribution of microinjected antisense oligonucleotides. Proc Natl Acad Sci USA 88:2702–2706
Leslie RA, Hunter AJ, Robertson HA (1999) Antisense oligonucleotide regulation of gene expression in the CNS: an overview. In: Leslie RA, Hunter AJ, Robertson HA (eds) Antisense technology in the central nervous system. Oxford University Press, New York, pp 1–7
Leto TL, Lomax KJ, Volpp DM, Nunoi H, Sechler JM, Nauseef WM, Clark RA, Gallin JI, Malech HL (1990) Cloning of a 67-kD neurtrophil oxidase factor with similarity to a non catalytic region of p60c-src. Science 248:727–730
Levin AA (1999) A review of the issues in the pharmacokinetics and toxicology of phosphorothioate antisense oligonucleotides. Biochim Biophys Acta 1489:69–84
Li Y, Breaker RR (1999) Kinetics of RNA degradation by specific base catalysis of transesterification involving the 2′ hydroxyl group. J Am Chem Soc 121:5364–5372
Li J, Zheng W, Kwon AH, Lu Y (2000) In vitro selection and characterization of a highly efficient Zn(II)-dependent RNA-cleaving deoxyribozyme. Nucleic Acids Res 28:481–488
Li J, Zhu D, Yi Z, He Y, Chun Y, Liu Y, Li N (2005) DNAzymes targeting the icl gene inhibit ICL expression and decrease Mycobacterium tuberculosis survival in macrophages. Oligonucleotides 15:215–222
Li J, Wang N, Luo Q, Wan L (2010) The 10–23 DNA enzyme generated by a novel expression vector mediate inhibition of taco expression in macrophage. Oliognucleotides 20:61–68
Liang Z, Wei S, Guan J, Luo Y, Gao J, Zhu H, Wu S, Liu T (2005) DNAzyme-mediated cleavage of surviving mRNA and inhibition of the growth of PANC-1 cells. J Gastroenterol Hepatol 20:1595–1602
Liu C, Cheng R, Sun LQ, Tien P (2001) Suppression of platelet-type 12-lipoxygenase activity in human erythroleukemia cell by an RNA-cleaving DNAzyme. Biochem Biophys Res Commun 284:1077–1082
Loke SL, Stein CA, Zhang XH, Mori K, Nakanishi M, Subasinghe C, Cohen JS, Neckers LM (1989) Characterization of oligonucleotide transport into living cells. Proc Natl Acad Sci USA 86:3474–3478
Lu ZX, Ye M, Yan GR, Li Q, Tang M, Lee LM, Sun LQ, Cao Y (2005) Effect of EBV LMP1 targeted DNAzymes on cell proliferation and apoptosis. Cancer Gene Ther 12:647–654
Marciano PG, Brettschneider J, Manduchi E, Davis JE, Eastman S, Raghupathi R, Saatman KE, Speed TP, Stoeckert CJ Jr, Eberwine JH, McIntosh TK (2004)Neuron-specific mRNA complexity responses during hippocampal apoptosis after traumatic brain injury. J Neurosci 24:2866-2876
McManus MT, Sharp PA (2002) Gene silencing in mammals by small interfering RNAs. Nat Rev Genet 3:737–747
McNamara JO, Andrechek ER, Wang Y, Viles KD, Rempel RE, Gilboa E, Sullenger BA, Giangrande PH (2006) Cell type-specific delivery of siRNA with aptamer-siRNA chimeras. Nat Biotechnol 24:1005–1015
Mitchell A, Dass CR, Sun LQ, Khachigian LM (2004) Inhibition of human breast carcinoma proliferation, migration, chemoinvasion and solid tumor growth by DNAzymes targeting the zinc finger transcription factor ERG-1. Nucleic Acids Res 32:3065–3069
Moilanen AM, Karvonen U, Poukka H, Yan W, Toppari J, Janne OA, Palvimo JJ (1999) A testis-specific androgen receptor coregulator that belongs to a novel family of nuclear protein. J Biol Chem 274:3700–3704
Moon LD, Asher RA, Rhodes KE, Fawcett JW (2001) Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC. Na Neurosci 4:465–466
Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W, Hartsough K, Machemer L, Radka S, Jadhav V, Vaish N, Zinnen S, Vargeese C, Bowman K, Shaffer CS, Jeffs LB, Judge A, MacLachlan I, Polisky B (2005) Potent and persistent in vivo anti-HBV activity of chemically modified siRNA. Nat Biotechnol 23:1002–1007
Nesic O, Svrakic NM, Xu GY, McAdoo D, Westlund KN, Hulsebosch CE, Ye Z, Galante A, Soteropoulos P, Tolias P, Young W, Hart RP, Perez-Polo JR (2002) DNA microarray analysis of the contused spinal cord: effect of NMDA receptor inhibiton. J Neurosci Res 15:406–423
O’Keefe J, Nadel L (1978) The hippocampus as a cognitive map. Oxford University Press, New York, pp 102–104
Orum H, Wengel J (2001) Locked nucleic acids: a promising molecular family for gene-funtions analysis and antisense drug development. Curr Opin Mol Ther 3:239–243
Patil SD, Rhodes DG, Burgess DJ (2004) DNA-based therapeutics and DNA delivery systems: a comprehensive review. AAPS J 7:E61–E77
Pawson T (1995) Protein modules and signalling networks. Nature 373:573–580
Pley HW, Lindes DS, DeLuca-Flaherty C, McKay DB (1993) Crystals of a hammerhead ribozyme. J Biol Chem 268:19656–19658
Pollak A (2011) Drugmakers’ fever for the power of RNA interference has cooled. New York Times, February 7 http://www.nytimes.com/2011/02/08/science/08rna.html?_r=2
Pun SH, Tack F, Bellocq NC, Cheng J, Grubbs BH, Jensen GS, Davies ME, Brewster M, Janicot M, Janssens B, Floren W, Bakker A (2004) Targeted delivery of RNA-cleaving DNA enzyme (DNAzyme) to tumor tissue by transferring-modified, cyclodextrin-based particles. Cancer Biol Ther 3:641–650
Resnick L, Fennell M (2004) Targeting JNK3 for the treatment of neurodegenerative disorders. Drug Discov Today 21:932–939
Ries A, Goldberg JL, Grimpe B (2007) A novel biological function for CD44 in axon growth of retinal ganglion cells indentified by a bioinformatics approach. J Neurochem 103:1491–1505
Roth A, Breaker RD (1998) An amino acid as a cofactor for a catalytic polynucleotide. Proc Natl Acad Sci USA 95:6027–6031
Sands H, Gorety-Feret LJ, Cocuzza AJ, Hobbs FW, Chidester D, Trainor GL (1994) Biodistribution and metabolism of internally 3H-labeled oligonucleotides. I. Comparison of a phosphodiester and a phosphorothioate. Mol Pharm 45:932–943
Santiago FS, Lowe HC, Kayurma MM, Chesterman CN, Baker A, Atkins DB, Khachigian LM (1999) New DNA enzyme targeting Erg-1 mRNA inhibits vascular smooth muscle proliferation and regrowth after injury. Nat Med 5:1264–1269
Santoro SW, Joyce GF (1997) A general purpose RNA-cleaving DNA enzyme. Proc Natl Acad Sci USA 94:4262–4266
Santoro SW, Joyce GF (1998) Mechanism and utility of an RNA-cleaving DNA enzyme. Biochemistry 37:13330–13342
Saville BJ, Collins RA (1990) A site-specific self-cleavage reaction performed by a novel RNA in Neurospora mitochondria. Cell 18:685–696
Scherr M, Morgan MA, Eder M (2003) Gene silencing mediated by small interfering RNAs in mammalian cells. Curr Med Chem 10:245–256
Schmidt C (2011) RNAi momentum fizzles as pharma shifts priorites. Nat Biotechnol 29:93–94
Schmidt D, Muller S (2002) Members of the PIAS family act as SUMO ligases for c-Jun and p53 and repress p53 activity. Proc Natl Acad Sci USA 99:2872–2877
Schubert S, Kurreck J (2004) Ribozyme- and deoxyribozyme-strategies for medical applications. Cur Drug Targets 5:667–681
Schubert S, Gul DC, Grunert HP, Zeichhardt H, Erdmann VA, Kurreck J (2003) RNA cleaving “10-23” DNAzymes with enhanced stability and activity. Nucleic Acids Res 31:5982–5992
Sel S, Wegmann M, Dicke T, Sel S, Henke W, Yildirin AO, Renz H, Garn H (2008) Effective prevention and therapy of experimental allergic asthma using a GATA-3 specific DNAzyme. J Allergy Clin Immunol 121:910–916
Shaw N, Arya DP (2008) Recognition of the unique structure of DNA:RNA hybrids. Biochimie 90:1026–1036
Shoji Y, Akhtar S, Periasamy A, Herman B, Juliano RL (1991) Mechanism of cellular uptake of modified oligodeoxynucleotides containing methylphosphonate linkages. Nucleic Acids Res 19:5543–5550
Shuai K, Liu B (2005) Regulation of gene-activation pathways by PIAS proteins in the immune system. Nat Rev Immunol 5:593–605
Silverman SK (2005) In vitro selection, characterization and application of deoxyribozymes that cleave RNA. Nucleic Acids Res 33:6151–6163
Simon MA, Bowtell DD, Dodson GS, Laverty TR, Rubin GM (1991) Ras1 and a putative guanine nucleotide exchange factor perform crucial steps in signaling by the sevenless protein tyrosine kinase. Cell 67:701–716
Sioud M, Leirdal M (2000) Design of nuclease resistant protein kinase C-alpha DNA enzyme with potential therapeutic application. J Mol Biol 296:937–947
Song E, Zhu P, Lee SK, Chowdhury D, Kussman S, Dykxhoorn DM, Feng Y, Palliser D, Weiner DB, Shankar P, Marasco WA, Lieberman J (2005) Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat Biotechnol 23:709–717
Sood V, Gupta N, Bano AS, Banerjea AC (2007a) DNA–enzyme-mediated cleavage of human immunodeficiency virus type 1 Gag RNA is significantly augmented by antisense-DNA molecules targeted to hybridize close to the cleavage site. Oligonucleotides 17:113–121
Sood V, Umwalla H, Gupta N, Chakraborti S, Banerjea AC (2007b) Potent knock down of HIV-1 replication by targeting HIV-1 Tat/Rev RNA sequences synergistically with catalytic RNA and DNA. AIDS 21:31–40
Sriram B, Banerjea AC (2000) In vitro-selected RNA cleaving DNA enzyme from a combinatorial library are potent inhibitors of HIV-1 gene expression. Biochem J 352:667–673
Stein CA, Cleary A, Yakubov L, Ledermann S (1993) Phosphorothioate oligodeoxy-nucleotides bind to the third variable loop domain (v3) of human immunodeficiency virus type 1 gp120. Antisense Res Dev 3:19–31
Sugimoto N, Okumoto Y, Ohmichi T (1999) Effect of metal ions and sequence of deoxyribozymes on their RA cleavage activity. J Chem Soc Perkin Trans 2:1381–1386
Sun LQ, Caims MJ, Gerlach WL, Withrington C, Wang L, King A (1999) Suppression of smooth muscle cell proliferation by a c-myc RNA-cleaving deoxyribozyme. J Biol Chem 274:17236–17241
Takahashi H, Abe T, Takai K, Takaku H (2000) Inhibition of influenza virus RNA (PB2 mRNA) expression by a modified DNA enzyme. Nucleic Acid Sym 44:287–288
Takahashi H, Hamazaki H, Habu Y, Hayashi M, Abe T, Miyano-Kurosaki N, Takaku H (2004) A new modified DNA enzyme that targets influenza virus A mRNA inhbitis viral infection in cultured cells. FEBS Lett 560:69–74
Tan X, Knesha R, Margolin W, Chen Y (2004) DNA enzyme generated by a novel single-stranded DNA expression vector inhibits expression of the essential bacterial cell division gene ftsZ. Biochemistry 43:1111–1117
Tator CH (1996) Experimental and clinical studies of the pathophysiology and management of acute spinal cord injury. J Spinal Cord Med 19:206–214
Tator CH (1998) Biological of neurological recovery and functional restoration after spinal cord injury. Neurosurgery 42:696–708
Thomson JB, Tuschl T, Eckstein F (1996) The hammerhead ribozyme. In: Eckstein F, Lilley DMJ (eds) Catalytic RNA. Springer, Berlin, pp 173–196
Trepanier J, Tanner JE, Momparler RL, Le ON, Alvarez F, Alfieri C (2006) Cleavage of intracellular hepatitis C RNA in the virus core protein coding region by deoxyribozymes. J Viral Hepat 13:131–138
Umwalla H, Chakraborti S, Sood V, Gupta N, Banerjea AC (2006) Potent inhibiton of HIV-1 gene expression and TAT-mediated apoptosis in human T cells by novel mono- and multitarget anti-TAT/Rev/Env ribozymes and a general purpose RNA-cleaving DNA-enzyme. Antiviral Res 72:134–144
Vester B, Hansen LH, Lundberg LB, Babu BR, Sorensen MD, Wengel J, Douthwaite S (2006) Locked nucleoside analogues expand the potential of DNAzymes to cleave structure RNA targets. BMC Mol Biol 7:19
Volpp BD, Nauseef WM, Donelson JE, Moser DR (1989) Cloning of the cDNA and functional expression of the 47 kilodalton cytosolic component of human neutrophil respiratory burst oxidase. Proc Natl Acad Sci USA 89:7195–7199
Walter AE, Turner DH, Kim J, Lyttle MH, Müller P, Mathews DH, Zuker M (1994) Coaxial stacking of helixes enhances binding of oligoribonucleotides and improves predictions of RNA folding. Proc Natl Acad Sci USA 91:9218–9222
Wang DZ, Hammond VE, Abud HE, Bertoncello I, McAvoy JW, Bowtell DD (1997) Mutation in Sos1 dominantly enhances a weak allele of the EGFR, demonstrating a requirement for Sos1 in EGFR signaling and development. Genes Dev 11:309–320
Wang DY, Lai BHY, Sen D (2002) A general strategy for effector-mediated control of RNA-cleaving ribozymes and DNA enzymes. J Mol Biol 318:33–43
Warashina M, Kuwabara T, Nakamatsu Y, Taira K (1999) Extremely high and specific activity of DNA enzymes in cells with a Philadelphia chromosome. Chem Biol 6:237–250
White J, Kielian M, Helenius A (1983) Membrane fusion proteins of enveloped animal viruses. Q Rev Biophys 16:151–195
Wo JE, Wu XL, Zhou LF, Yao HP, Chen LW, Dennin RH (2005) Effective inhibition of expression of hepatitis B virus genes by DNAzymes. World J Gastroenterol 11:3504–3507
Wu HN, Lin YJ, Lin FP, Makino S, Chang MF, Lai MM (1989) Human hepatitis delta virus RNA subfragments contain an autocleavage activity. Proc Natl Acad Sci USA 86:1831–1835
Wu S, Xu J, Liu J, Yan X, Zhu X, Xiao G, Sun L, Tien P (2007) An efficient RNA-cleaving DNA enzyme can specifically target the 5′-untranslated region of severe acute respiratory syndrome associated coronavirus (SARS-CoV). J Gene Med 9:1080–1086
Wu Y, Han W, Liu GN (2010) A DNA enzyme targeting Egr-1 inhibitis rat vascular smooth muscle cell proliferation by down-regulation of cyclin D1 and TGF-beta 1. Braz J Med Biol Res 43:17–24
Xiao Y, Allen EC, Silverman SK (2010) Merely two mutations switch a DNA-hydrolyzing deoxyribozyme form heterobimetallic (Zn2+/Mn2+) to monometallic (Zn2+−only) behavior. Chem Commun 47:1749–1751
Yakubov L, Khaled Z, Zhang LM, Truneh A, Vlassov V, Stein CA (1993) Oligodeoxy-nucleotides interact with recombinant CD4 at multiple sites. J Biol Chem 268:18818–18823
Yang L, Xiao L, Ma X, Tang M, Weng X, Chen X, Sun L, Cao Y (2009) Effect of DNAzymes targeting Akt1 on cell proliferation and apoptosis in nasopharyngeal carcinoma. Cancer Biol Ther 8:366–371
Yu H, Chen JK, Feng S, Dalgamo DC, Brauer AW, Schreiber SL (1994) Structural basis for the binding of proline-rich peptides to SH3 domains. Cell 76:933–945
Yuan BF, Xue Y, Luo M, Hao YH, Tan Z (2007) Two DNAzymes targeting the telomerase mRNA with large difference in Mg2+ concentration for maximal catalytic activity. Int J Biochem Cell Biol 39:1119–1129
Zaborowska Z, Fürste JP, Erdmann VA, Kurreck J (2002) Sequence requirements in the catalytic core of the “10-23” DNA eEnzyme. J Biol Chem 277:40617–40622
Zamecnik PC, Stephenson ML (1978) Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc Natl Acad Sci USA 75:280–284
Zhang L, Gasper WJ, Stass SA, Ioffe OB, Davis MA, Mixson AJ (2002) Angiogenic inhibition mediated by a DNAzyme that targets vascular endothelial growth factor receptor 2. Cancer Res 62:5463–5469
Zhang G, Dass CR, Sumithran E, Di Girolamo N, Sun LQ, Khachigian LM (2004) Effect of deoxyribozymes targeting c-Jun on solid tumor growth and angiogenesis in rodents. J Natl Cancer Inst 96:683–696
Zhou J, Yang XQ, Xie YY, Zhao XD, Jiang LP, Wang LJ, Cui YX (2007) Inhibition of respiratory syncytial virus of subgroups A and B using deoxyribozyme DZ1133 in mice. Virus Res 130:241–248
Author information
Authors and Affiliations
Corresponding author
Additional information
Research on deoxyribozymes and systems biology in the Grimpe laboratory has been supported by the German Research Foundation (DFG), Forschungskommission der HHU, Wilson Medical Research Foundation, Glaucoma Foundation and Florida State Fund.
Rights and permissions
About this article
Cite this article
Grimpe, B. Deoxyribozymes and bioinformatics: complementary tools to investigate axon regeneration. Cell Tissue Res 349, 181–200 (2012). https://doi.org/10.1007/s00441-011-1291-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-011-1291-6