Abstract
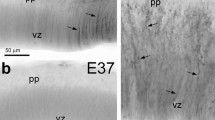
The successful regrowth of retinal ganglion cell (RGC) axons after optic nerve (ON) axotomy in Gallotia galloti indicates a permissive role of the glial environment. We have characterised the astroglial lineage of the lizard optic pathway throughout its ontogeny (embryonic stage 30 [E30] to adults) by using electron microscopy and immunohistochemistry to detect the proliferation marker PCNA (proliferating cell nuclear antigen), the transcription factor Pax2 and the gliofilament proteins vimentin (Vim) and GFAP (glial fibrillary acidic protein). PCNA+ cells were abundant until E39, with GFAP+/PCNA+ astrocytes being observed between E37 and hatching. Proliferation diminished markedly afterwards, being undetectable in the adult optic pathway. Müller glia of the central retina expressed Pax2 from E37 and their endfeet accumulated Vim from E33 and GFAP from E37 onwards. Astrocytes were absent in the avascular lizard retina, whereas abundant Pax2+ astrocytes were observed in the ON from E30. A major subpopulation of these astrocytes coexpressed Vim from E35 and also GFAP from E37 onwards; thus the majority of mature astrocytes coexpressed Pax2/Vim/GFAP. The astrocytes were ultrastructurally identified by their gliofilaments, microtubules, dense bodies, desmosomes and glycogen granules, which preferentially accumulated in cell processes. Astrocytes in the adult ON coexpressed both gliofilaments and presented desmosomes indicating a reinforcement of the ON structure; this is physiologically necessary for local adaptation to mechanical forces linked to eye movement. We suggest that astrocytes forming this structural scaffold facilitate the regrowth of RGCs after ON transection.





Similar content being viewed by others
Abbreviations
- CN:
-
Caudal optic nerve
- CPONJ:
-
Conus papillaris-optic nerve junction
- CR:
-
Central retina
- GFAP:
-
Glial fibrillary acidic protein
- INL:
-
Inner nuclear layer
- MN:
-
Mid optic nerve
- OCh:
-
Optic chiasm
- ON:
-
Optic nerve
- ONH:
-
Optic nerve head
- OTr:
-
Optic tract
- PCNA:
-
Proliferating cell nuclear antigen
- PR:
-
Peripheral retina
- RGC:
-
Retinal ganglion cell
- RONJ:
-
Retina-optic nerve junction
- Vim:
-
Vimentin
References
Alfayate MC, Santos E, Yanes C, Casañas N, Viñoly R, Romero-Alemán MM, Monzón-Mayor M (2011) Ontogeny of the conus papillaris of the lizard G. galloti and cellular response following transection of the optic nerve. Immunohistochemical and ultrastructural study. Cell Tissue Res 344:63–83
Alvarez-Bolado G, Schwarz M, Gruss P (1997) Pax-2 in the chiasm. Cell Tissue Res 290:197–200
Bixby JL, Harris WA (1991) Molecular mechanisms of axon growth and guidance. Annu Rev Cell Biol 7:117–159
Bodega G, Suarez I, Rubio M, Fernandez B (1995) Type II cytokeratin expression in adult vertebrate spinal cord. Tissue Cell 27:555–559
Boije H, Ring H, López-Gallardo M, Prada C, Hallböök F (2010) Pax2 is expressed in a subpopulation of Müller cells in the central chick retina. Dev Dyn 239:1858–1866
Brodkey JA, Gates MA, Laywell ED, Steindler DA (1993) The complex nature of interactive neuroregeneration-related molecules. Exp Neurol 123:251–270
Calvo JL, Carbonell AL, Boya J (1990) Coexpression of vimentin and glial fibrillary acidic protein in astrocytes of the adult rat optic nerve. Brain Res 532:355–357
Chan-Ling T, Stone J (1991) Factors determining the morphology and distribution of astrocytes in the cat retina: a contact-spacing model of astrocyte interaction. J Comp Neurol 303:387–399
Chu Y, Hughes S, Chan-Ling T (2001) Differentiation and migration of astrocyte precursor cells and astrocytes in human fetal retina: relevance to optic nerve coloboma. FASEB J 15:2013–2015
Cohen I, Sivron T, Lavie V, Blaugrund E, Schwartz M (1994) Vimentin immunoreactive glial cells in the fish optic nerve: implications for regeneration. Glia 10:16–29
Dahl D, Rueger DC, Bignami A, Weber K, Osborn M (1981) Vimentin, the 57 000 molecular weight protein of fibroblast filaments, is the major cytoskeletal component in immature glia. Eur J Cell Biol 24:191–196
Dakubo GD, Beug ST, Mazerolle CJ, Thurig S, Wang Y, Wallace VA (2008) Control of glial precursor cell development in the mouse optic nerve by sonic hedgehog from retinal ganglion cells. Brain Res 1228:27–42
Dávila JC, Guirado S, De La Calle A, Marín-Girón F (1987) The intra-ocular portion of the turtle Mauremys caspica. J Anat 151:189–198
De Guevara R, Pairault C, Pinganaud G (1994) Expression of vimentin and GFAP and development of the retina in the trout. C R Acad Sci III 317:737–741
Doh ST, Hao H, Loh SC, Patel T, Tawil HY, Chen DK, Pashkova A, Shen A, Wang H, Cai L (2010) Analysis of retinal cell development in chick embryo by immunohistochemistry and in ovo electroporation techniques. BMC Dev Biol 10:8–24
Dufaure JP, Hubert J (1961) Table de dévelopment du lizard viviparae (Lacerta vivipara jacquin). Arch Anat Microsc Morphol Exp 50:309–327
Dunlop SA, Tee LB, Stirling RV, Taylor AL, Runham PB, Barber AB, Kuchling G, Rodger J, Roberts JD, Harvey AR, Beazley LD (2004) Failure to restore vision after optic nerve regeneration in reptiles: interspecies variation in response to axotomy. J Comp Neurol 478:292–305
Fischer AJ, Zelinka C, Scott MA (2010) Heterogeneity of glia in the retina and optic nerve of birds and mammals. PLoS ONE 5:e10774
Fujita Y, Imagawa T, Uehara M (2001) Fine structure of the retino-optic nerve junction in the chicken. Tissue Cell 33:129–134
Galou M, Colucci-Guyon E, Ensergueix D, Ridet JL, Gimenez y Rivotta M, Privat A, Babinet C, Dupouey P (1996) Disrupted glial fibrillary acidic protein network in astrocytes from vimentin knockout mice. J Cell Biol 133:853–863
Gerhardt H, Schuck J, Wolburg H (1999) Differentiation of a unique macroglial cell type in the pecten oculi of the chicken. Glia 28:201–214
Hirata A, Kitaoka T, Isihigooka H, Ueno S (1991) Cytochemical studies of transitional area between retina and optic nerve. Acta Ophthalmol (Copenh) 69:71–75
Holen T (2011) The ultrastructure of lamellar stack astrocytes. Glia 59:1075–1083
Jadhav AP, Roesch K, Cepko CL (2009) Development and neurogenic potential of Muller glial cells in the vertebrate retina. Prog Retin Eye Res 28:249–262
Jeffery G (2001) Architecture of the optic chiasm and the mechanisms that sculpt its development. Physiol Rev 81:1393–1414
Jeffery G, Levitt JB, Cooper HM (2008) Segregated hemispheric pathways through the optic chiasm distinguish primates from rodents. Neuroscience 157:637–643
Jonas JB, Mardin CY, Schlotzer-Schrehardt U, Naumann GO (1991) Morphometry of the human lamina cribrosa surface. Invest Ophthalmol Vis Sci 32:401–405
Kálmán M, Székely AD, Csillag A (1998) Distribution of glial fibrillary acidic protein and vimentin-immunopositive elements in the developing chicken brain from hatch to adulthood. Anat Embryol (Berl) 198:213–235
Kimelberg HK (2010) Functions of mature mammalian astrocytes: a current view. Neuroscientist 16:79–106
Kreft M, Potokar M, Stenovec M, Pangrsic T, Zorec R (2009) Regulated exocytosis and vesicle trafficking in astrocytes. Ann N Y Acad Sci 1152:30–42
Lang DM, Monzón-Mayor M, Bandtlow CE, Stuermer CAO (1998) Retinal axon regeneration in the lizard Gallotia galloti in the presence of CNS myelin and oligodendrocytes. Glia 23:61–74
Lang DM, Romero-Alemán MM, Arbelo-Galván JF, Stuermer CA, Monzón-Mayor M (2002) Regeneration of retinal axons in the lizard Gallotia galloti is not linked to generation of new retinal ganglion cells. J Neurobiol 52:322–335
Lang DM, Monzón-Mayor M, Romero-Alemán MM, Yanes C, Santos E, Pesheva P (2008) Tenascin-R and axon growth-promoting molecules are up-regulated in the regenerating visual pathway of the lizard (Gallotia galloti). Dev Neurobiol 68:899–916
Lara JM, Velasco A, Lillo C, Jimeno D, Aijon J (1998) Characterization of the glial cells in the teleost visual pathway. In: Bernardo-Castellano (ed) Understanding glial cells. Kluwer Academic, Dordrecht, pp 3–18
Lepekhin EA, Eliasson C, Berthold CH, Berezin V, Bock E, Pekny M (2001) Intermediate filaments regulate astrocyte motility. J Neurochem 79:617–625
Levine RL (1989) Organization of astrocytes in the visual pathways of the goldfish: an immunohistochemical study. J Comp Neurol 285:231–245
Levine RL (1993) Axon dependent glial changes during optic fiber regeneration in the goldfish. J Comp Neurol 333:543–553
Lewis GP, Matsumoto B, Fisher SK (1995) Changes in the organization and expression of cytoskeletal proteins during retinal degeneration induced by retinal detachment. Invest Ophthalmol Vis Sci 36:2404–2416
Lillo C, Velasco A, Jimeno D, Cid E, Lara JM, Aijon J (2002) The glial design of a teleost optic nerve head supporting continuous growth. J Histochem Cytochem 50:1289–1302
Lundkvist A, Reichenbach A, Betsholtz C, Carmeliet P, Wolburg H, Pekny M (2004) Under stress, the absence of intermediate filaments from Muller cells in the retina has structural and functional consequences. J Cell Sci 117:3481–3488
Macdonald R, Scholes J, Strähle U, Brennan C, Holder N, Brand M, Wilson SW (1997) The Pax protein Noi is required for commissural axon pathway formation in the rostral forebrain. Development 124:2397–2408
Maggs A, Scholes J (1986) Glial domains and nerve fiber patterns in the fish retinotectal pathway. J Neurosci 6:424–438
Maggs A, Scholes J (1990) Reticular astrocytes in the fish optic nerve: macroglia with epithelial characteristics form an axially repeated lacework pattern, to which nodes of Ranvier are apposed. J Neurosci 10:1600–1614
Menet V, Prieto M, Privat A, Giménez-Ribotta M (2003) Axonal plasticity and functional recovery after spinal cord injury in mice deficient in both glial fibrillary acidic protein and vimentin genes. Proc Natl Acad Sci USA 100:8999–9004
Miller RH, Ffrench-Constant C, Raff MC (1989a) The macroglial cells of the rat optic nerve. Annu Rev Neurosci 12:517–534
Miller RH, Fulton BP, Raff MC (1989b) A novel type of glial cell associated with nodes of Ranvier in rat optic nerve. Eur J Neurosci 1:172–180
Mitashov VI (2001) Multipotent and stem cells in the developing, definitive, and regenerating vertebrate eye. Izv Akad Nauk Ser Biol 6:717–727
Monzón-Mayor M, Yanes C, Ghandour MS, De Barry J, Gombos G (1990a) Glial fibrillary acidic protein and vimentin immunohistochemistry in the developing and adult midbrain of the lizard Gallotia galloti. J Comp Neurol 295:569–579
Monzón-Mayor M, Yanes C, James JG, Sturrock RR (1990b) An ultrastructural study of the development of astrocytes in the midbrain of the lizard. J Anat 170:33–41
Monzón-Mayor M, Yanes C, Tholey G, De Barry J, Gombos G (1990c) Immunohistochemical localization of glutamine synthetase in mesencephalon and telencephalon of the lizard Gallotia galloti during ontogeny. Glia 3:81–97
Monzón-Mayor M, Yanes-Méndez C, De Barry J, Capdevilla-Carbonell C, Renau-Piqueras J, Tholey G, Gombos G (1998) Heterogeneous immunoreactivity of glial cells in the mesencephalon of a lizard: a double labeling immunohistochemical study. J Morphol 235:109–119
Morcos Y, Chan-Ling T (2000) Concentration of astrocytic filaments at the retinal optic nerve junction is coincident with the absence of intra-retinal myelination: comparative and developmental evidence. J Neurocytol 29:665–678
Onteniente B, Kimura H, Maeda T (1983) Comparative study of the glial fibrillary acidic protein in vertebrates by PAP immunohistochemistry. J Comp Neurol 215:427–436
Oyama T, Abe H, Ushiki T (2006) The connective tissue and glial framework in the optic nerve head of the normal human eye: light and scanning electron microscopic studies. Arch Histol Cytol 69:341–356
Parrilla M, Lillo C, Herrero-Turrion MJ, Arévalo R, Lara JM, Aijón J, Velasco A (2009) Pax2 in the optic nerve of the goldfish, a model of continuous growth. Brain Res 1255:75–88
Pereira A Jr, Furlan FA (2010) Astrocytes and human cognition: modelling information integration and modulation of neuronal activity. Prog Neurobiol 92:405–420
Potokar M, Kreft M, Li L, Daniel Andersson J, Pangrsic T, Chowdhury HH, Pekny M, Zorec R (2007) Cytoskeleton and vesicle mobility in astrocytes. Traffic 8:12–20
Prada FA, Espinar A, Chmielewski CE, Dorado ME, Genis-Gálvez JM (1989) Regional adaptation of Muller cells in the chick retina. A Golgi and electron microscopical study. Histol Histopathol 4:309–315
Quesada A, Prada FA, Aguilera Y, Espinar A, Carmona A, Prada C (2004) Peripapillary glial cells in the chick retina: a special glial cell type expressing astrocyte, radial glia, neuron, and oligodendrocyte markers throughout development. Glia 46:346–355
Quitschke W, Schechter N (1986) Homology and diversity between intermediate filament proteins of neuronal and nonneuronal origin in goldfish optic nerve. J Neurochem 46:545–555
Raff MC (1989) Glial cell diversification in the rat optic nerve. Science 243:1450–1455
Ramos-Steffens A (1980) Tabla del desarrollo embrionario de la lacerta Gallotia galloti (período de organogénesis), y aspectos de su reproducción. Minor thesis, Facultad de Biología, Universidad de La Laguna, España
Reese BE, Maynard TM, Hocking DR (1994) Glial domains and axonal reordering in the chiasmatic region of the developing ferret. J Comp Neurol 349:303–324
Romero-Alemán MM, Monzón-Mayor M, Yanes C, Arbelo-Galván JF, Lang D, Renau-Piqueras J, Negrín-Martínez C (2003) S100 immunoreactive glial cells in the forebrain and midbrain of the lizard Gallotia galloti during ontogeny. J Neurobiol 57:54–66
Romero-Alemán MM, Monzón-Mayor M, Yanes C, Lang D (2004) Radial glial cells, proliferating periventricular cells, and microglia might contribute to successful structural repair in the cerebral cortex of the lizard Gallotia galloti. Exp Neurol 188:74–85
Romero-Alemán MM, Monzón-Mayor M, Santos E, Yanes C (2010) Expression of neuronal markers, synaptic proteins, and glutamine synthetase in the control and regenerating lizard visual system. J Comp Neurol 518:4067–4087
Rubin LL, Staddon JM (1999) The cell biology of the blood-brain barrier. Annu Rev Neurosci 22:11–28
Rungger-Brändle E, Achtstätter T, Franke WW (1989) An epithelium-type cytoskeleton in a glial cell: astrocytes of amphibian optic nerves contain cytokeratin filaments and are connected by desmosomes. J Cell Biol 109:705–716
Santos E, Yanes CM, Monzón-Mayor M, del Mar Romero-Alemán M (2006) Peculiar and typical oligodendrocytes are involved in an uneven myelination pattern during the ontogeny of the lizard visual pathway. J Neurobiol 66:1115–1124
Santos E, Monzón-Mayor M, Romero-Alemán MM, Yanes C (2008) Distribution of neurotrophin-3 during the ontogeny and regeneration of the lizard (Gallotia galloti) visual system. Dev Neurobiol 68:31–44
Sassoè Pognetto M, Panzanelli P, Artero C, Fasolo A, Cantino D (1992) Comparative study of glial fibrillary acidic protein (GFAP) like immunoreactivity in the retina of some representative vertebrates. Eur J Histochem 36:467–477
Scholes J (1991) The design of the optic nerve in fish. Vis Neurosci 7:129–139
Skoff R, Price DL, Stocks SA (1976) Electron microscopic autoradiographic studies of gliogenesis in rat optic nerve. II. Time of origin. J Comp Neurol 169:313–334
Stanke J, Moose HE, El-Hodiri HM, Fischer AJ (2010) Comparative study of Pax2 expression in glial cells in the retina and optic nerve of birds and mammals. J Comp Neurol 518:2316–2333
Stensaas LJ (1977) The ultrastructure of astrocytes, oligodendrocytes, and microglia in the optic nerve of urodele amphibians (A. punctatum, T. pyrrhogaster, T. viridescens). J Neurocytol 6:269–286
Sturrock RR (1975) A light and electron microscopic study of proliferation and maduration of fibrous astrocytes in the optic nerve of human embryo. J Anat 119:223–234
Suárez I, Raff MC (1989) Subpial and perivascular astrocytes associated with nodes of Ranvier in the rat optic nerve. J Neurocytol 18:577–582
Tekkök SB, Brown AM, Westenbroek R, Pellerin L, Ransom BR (2005) Transfer of glycogen-derived lactate from astrocytes to axons via specific monocarboxylate transporters supports mouse optic nerve activity. J Neurosci Res 81:644–652
Thanos S, Püttmann S, Naskar R, Rose K, Langkamp-Flock M, Paulus W (2004) Potential role of Pax-2 in retinal axon navigation through the chick optic nerve stalk and optic chiasm. J Neurobiol 59:8–23
Torres M, Gómez-Pardo E, Gruss P (1996) Pax2 contributes to inner ear patterning and optic nerve trajectory. Development 22:3381–3391
Triviño A, Ramírez JM, Salazar JJ, Ramírez AI, García-Sánchez J (1996) Immunohistochemical study of human optic nerve head astroglia. Vision Res 36:2015–2028
Vaughn JE (1969) An electron microscopic analysis of gliogenesis in rat optic nerve. Z Zell Mikrosk Anat 94:293–327
Williams SE, Mann F, Erskine L, Sakurai T, Wei S, Rossi DJ, Gale NW, Holt CE, Mason CA, Henkemeyer M (2003) Ephrin-B2 and EphB1 mediate retinal axon divergence at the optic chiasm. Neuron 39:919–935
Wolburg H, Bäuerle C (1993) Astrocytes in the lamina cribrosa of the rat optic nerve: are their morphological peculiarities involved in an altered blood-brain barrier? J Hirnforsch 34:445–459
Won MH, Kang TC, Cho SS (2000) Glial cells in the bird retina: immunochemical detection. Microsc Res Tech 50:151–160
Yanes C, Monzón-Mayor M, Ghandour MS, De Barry J, Gombos G (1990) Radial glia and astrocytes in developing and adult telencephalon of the lizard Gallotia galloti as revealed by immunohistochemistry with anti-GFAP and anti-Vimentin antibodies. J Comp Neurol 295:559–568
Ye H, Hernández MR (1995) Heterogeneity of astrocytes in human optic nerve head. J Comp Neurol 362:441–452
Acknowledgements
The technical assistance of the Electron Microscopy Services of the University of La Laguna (ULL) and the University of Las Palmas de Gran Canaria (ULPGC) are greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the Spanish Ministry of Education (Research Project BFU2007-67139) and the Regional Canary Islands Government (ACIISI, Research Project SolSubC200801000281; Project ULPAPD-08/01-4).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Panoramic views of the retina (a, b) and optic nerve-optic tract (ON-OTr; c, d) of adult (Ad) lizards immunolabelled for vimentin (Vim) and glial fibrillary acidic protein (GFAP). a, b Immunofluorescence labelling for Vim (a) and immunoperoxidase staining for GFAP (b) in the retina. Müller glia endfeet are weakly Vim+ in the central (CR) compared with the peripheral retina (PR), whereas they show homogeneous GFAP labelling along the entire retina (l lens). c, d Immunoperoxidase staining for Vim (c) and GFAP (d) in the ON-OTr. Note intense Vim staining in the caudal nerve (CN) and strong GFAP staining in the midnerve (MN). Bars 250 μm (JPEG 90 kb)
Rights and permissions
About this article
Cite this article
Casañas, M.N., Santos, E., Yanes, C. et al. Development of astroglia heterogeneously expressing Pax2, vimentin and GFAP during the ontogeny of the optic pathway of the lizard (Gallotia galloti): an immunohistochemical and ultrastructural study. Cell Tissue Res 345, 295–311 (2011). https://doi.org/10.1007/s00441-011-1211-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-011-1211-9